Back
Purpose: Designing novel drug delivery systems is key to enable new pharmaceutical products to improve patient health by enhancing the delivery of a therapeutic to its target, improving its safety, and facilitating patient compliance [1, 2]. The development of new “intelligent” materials has advanced the field of emerging technologies such as enzyme-responsive drug delivery systems [3, 4]. In particular, matrix metalloproteinase (MMP) responsive hydrogels represent promising opportunities for intradermal on-demand delivery due to the hydrated network where enzymes can come in close proximity and interact with the sensitive material or the incorporated sensitive moiety [5-9]. Here, we report MMP-responsive hydrogels formed by step-growth photopolymerization of biscysteine peptide linkers with norbornene functionalized branched polyethylene glycols for the treatment of inflammatory skin diseases. To validate the MMP-responsive hydrogels for their intended use they were loaded with a Janus kinase (JAK) inhibitor tofacitinib citrate. The MMP responsiveness and the biocompatibility of the hydrogels were evaluated, and the bioactivity of the released tofacitinib citrate was investigated in an atopic dermatitis (AD) like keratinocyte assay.
Methods: 1H high resolution magic angle spinning nuclear magnetic resonance (HR-MAS NMR) spectroscopy (Bruker Avance 600 HD III, 14.1 T) was used to investigate the conversion of norbornenes and thiols to thioethers via the disappearance alkene protons peaks of norbornene under hydrated conditions. In vitro release studies were performed using Franz Diffusion cells with a nitrocellulose membrane (molecular cut-off: 9 kDa). Tofacitinib citrate was quantified using HPLC (Waters ACQUITY UPLC H-class system, Massachusetts, US). The biocompatibility study was conducted with fibroblasts (murine 3T3) and the anti-inflammatory activity of tofacitinib citrate released from the hydrogels was verified employing human epidermal keratinocytes which was stimulated with a cocktail of T-cell cytokines characteristic of the inflammatory skin disease AD (IL-4, IL-13, IL-22 and INF-γ) [10].
Results: 1H HR-MAS NMR enabled quantitative investigation of the degree of cross-linking in hydrated multi-arm PEG hydrogels after photopolymerization. Firstly, we found that the photo-initiator LAP was more efficient than Irgacure 2959, resulting in a ~100% disappearance of the C=C double bond in the multi-arm PEG functionalized NB groups within 30 s (Figure 1A). Secondly, we noted that the degree of cross-linking changed remarkably with the PEG polymer concentration (Figure 1B). Irrespective of the cross-linker, almost complete cross-linking was seen for hydrogels with polymer concentrations of 5% and 10% (w/w) of both 4-arm and 8-arm PEG hydrogels, respectively. In the absence of MMP stimulus, a cumulative release of 32% and 25% of the total amount of tofacitinib citrate in the hydrogels was seen after 48 h incubation for 4-arm and 8-arm MMP-responsive PEG hydrogels, respectively. Upon addition of 20 nM MMP-9, the cumulative release of tofacitinib citrate reached 67% and 49% for 4-arm and 8-arm PEG hydrogels, respectively (Figure 2A). As a control, the non-responsive 4-arm and 8-arm PEG hydrogels were tested in parallel, and no significant difference in the cumulative release was found between MMP-9-exposed hydrogels and controls (Figure 2B). The cumulative release observed in the absence of MMP-9 was lower for the 8-arm than for 4-arm hydrogels cross-linked (Figure 2C-D). To mimic the flare-up characteristics seen in several inflammatory skin diseases and to investigate the enzymatic release associated with such flare-ups, activated MMP-9 was repeatedly. Both 4-arm and 8-arm PEG hydrogels were found to respond to the addition of newly activated MMP-9 stimulus, as confirmed by the stepwise increase in cumulative release (Figure 2E). Next, we investigated the cytotoxicity of hydrogel breakdown products after exposure to MMP-9 (Figure 3A), as well as to all separate hydrogel components employing 3T3 fibroblasts (Figure 3B). No significant reduction in cell viability was observed for either the MMP-9 generated cleavage products or the separate hydrogel components. In the AD keratinocyte assay it was found that samples collected from experiments without MMP-9 do not inhibit the production of CCL-2 in the early sampling times ( < 1 h) for 4-arm PEG (Figure 3C). When the hydrogels were exposed to 20nM MMP-9, however, the resulting increased tofacitinib citrate concentration present after triggered release was found to result in full inhibition of CCL-2 production at all sampling times for 4-arm PEG hydrogels (Figure 3D).
Conclusion: Here, we have presented a rational design of MMP-responsive hydrogels formed by thiol-norbornene photopolymerization for the treatment of inflammatory skin disease. As a pre-clinical proof-of-concept, we verified that such hydrogels can be designed to deliver tofacitinib citrate in response to MMP-9 exposure. Under the conditions investigated, no cytotoxicity was observed, neither related to the MMP-responsive hydrogels nor to the cleavage products generated during their exposure to MMP-9. In an AD-like keratinocyte assay, we further confirmed that the incorporation of tofacitinib citrate into the hydrogels does not compromise the anti-inflammatory effect of tofacitinib citrate. Translation into an intradermal depot of such hydrogels seems to be promising with respect to the treatment of flare-up driven diseases characterized by high MMP activity.
References: 1. May, M., Why drug delivery is the key to new medicines. Nat Med, 2022. 21(6): p. 1100-1102.
2. Lu, Y., et al., Bioresponsive materials. Nature Reviews Materials, 2016. 2(1).
3. Fairbanks, B.D., et al., A Versatile Synthetic Extracellular Matrix Mimic via Thiol-Norbornene Photopolymerization. Adv Mater, 2009. 21(48): p. 5005-5010.
4. Shahriari, M., et al., Enzyme responsive drug delivery systems in cancer treatment. J Control Release, 2019. 308: p. 172-189.
5. Sobczak, M., Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems-State of Knowledge and Future Prospects. Int J Mol Sci, 2022. 23(8).
6. Yao, Q., et al., MMP-Responsive 'Smart' Drug Delivery and Tumor Targeting. Trends Pharmacol Sci, 2018. 39(8): p. 766-781.
7. Guo, J.W. and S.H. Jee, Strategies to Develop a Suitable Formulation for Inflammatory Skin Disease Treatment. Int J Mol Sci, 2021. 22(11).
8. Stewart-McGuinness, C., et al., Defining the Protease and Protease Inhibitor (P/PI) Proteomes of Healthy and Diseased Human Skin by Modified Systematic Review. Biomolecules, 2022. 12(3).
9. Eckhard, U., et al., Active site specificity profiling of the matrix metalloproteinase family: Proteomic identification of 4300 cleavage sites by nine MMPs explored with structural and synthetic peptide cleavage analyses. Matrix Biol, 2016. 49: p. 37-60.
10. Tine Skak-Nielsen , N.W.H., Inhibition of CCL2 release from IL-4, IL-13, IL-22 and IFNg-stimulated primary human keratinocytes. LEO Pharma Open Innovation, 2020.
Acknowledgments: The authors acknowledge Dan Buchvardt from Quality Laboratories Denmark, LEO Pharma A/S for the support with the mechanical compression plate testing and Birgitte Karna Davidsen from In Vitro Biology, LEO Pharma A/S for assistance with the cell handling of keratinocytes and fibroblast.
Funding: The work reported in this paper was financially supported by LEO Pharma A/S (2750 Ballerup, Denmark), as well as the LEO Foundation Center for Cutaneous Drug Delivery, University of Copenhagen (Grant number 2016-11-01; AH, MM)
Declaration of interest: The authors declare no conflicts of interest.
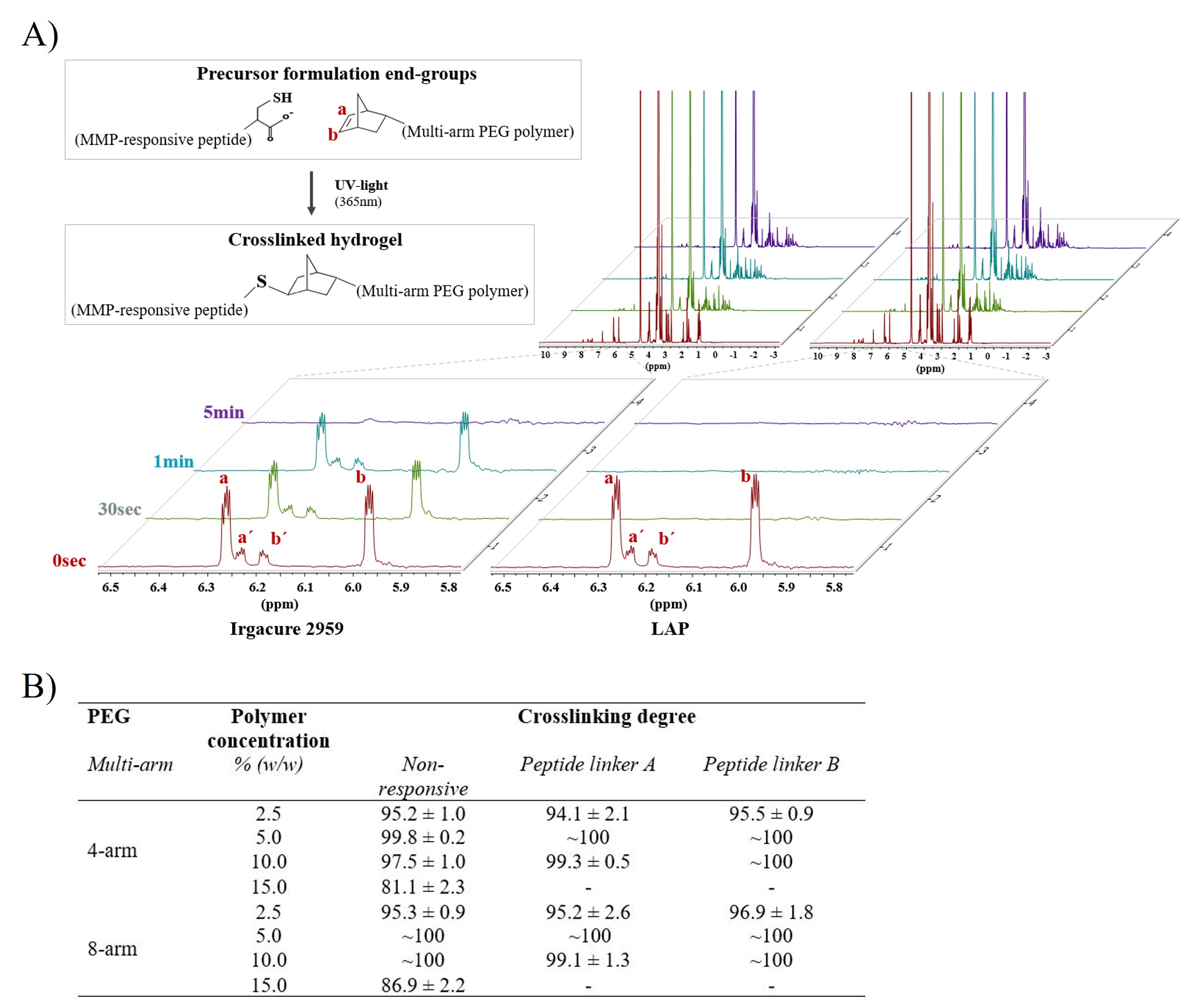
Figure 1. (A) Investigation of cross-linking degree in hydrated PEG hydrogels using 1H HR-MAS NMR spectroscopy. 1H NMR spectra for formulations containing Irgacure (0.05%, w/w) and LAP (0.05%, w/w) are shown. The protons originating from the double bond in norbornene can be detected between 5.95 and 6.30 ppm. (B) 1H HR-MAS NMR spectroscopy results on the cross-linking degree for hydrogels prepared using non-responsive linker, peptide linker A, and peptide linker B. The hydrogels were crosslinked by UV light (365 nm) for 30 s. N = 3.
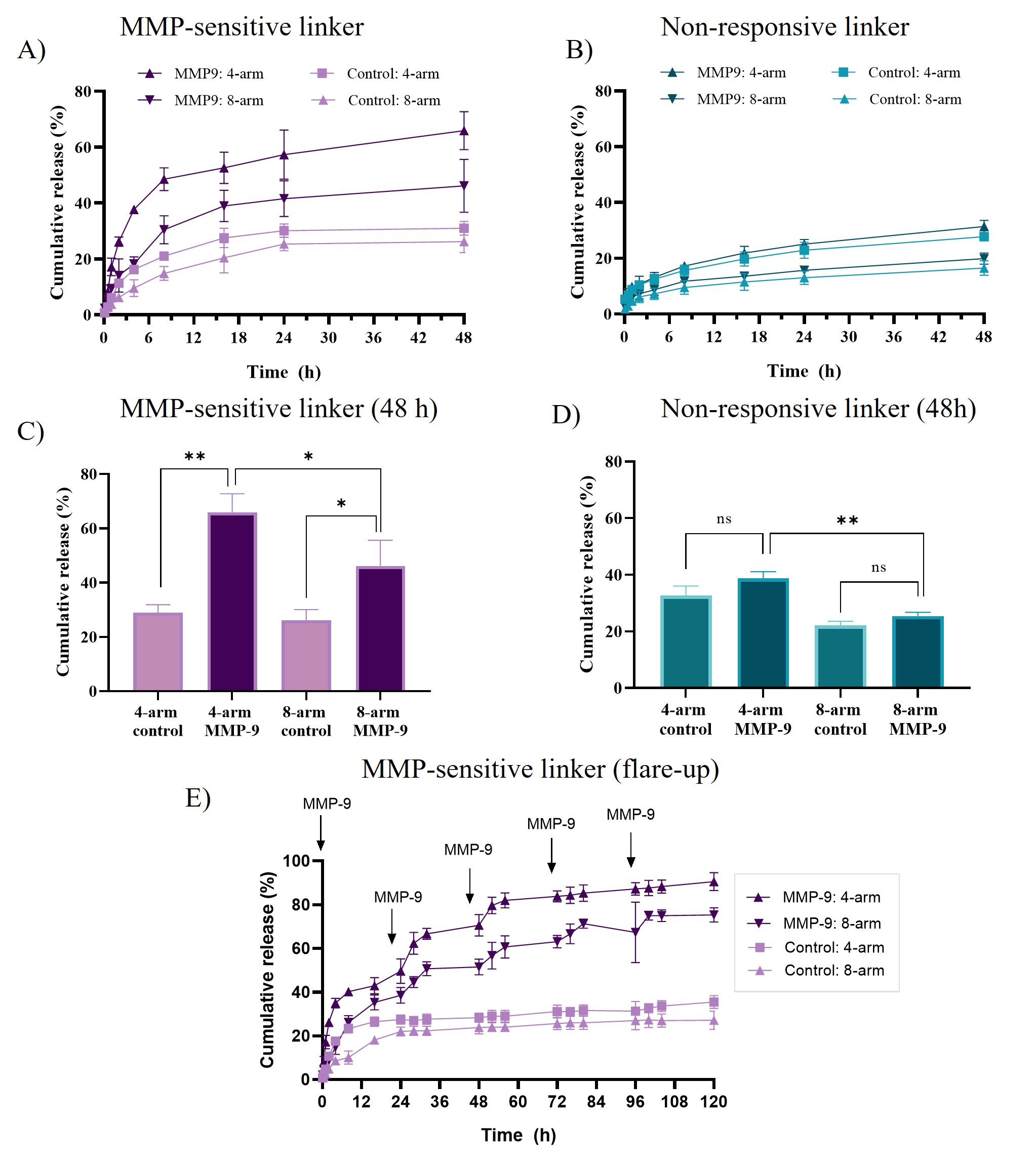
Figure 2. Cumulative release of tofacitinib citrate from 5% (w/w) 4-arm and 8-arm PEG (w/w) hydrogels in PBS at 37 °C without and with 20 nM activated MMP-9, respectively. Results are shown for PEG hydrogels cross-linked with (A) MMP-sensitive linker B and (B) non-responsive linker. The MMP-responsive cumulative drug release is shown after 48 h for hydrogels for (C) MMP-sensitive linker B and (D) MMP-insensitive linker. (E) Cumulative drug release after repeated MMP-9 exposure (↓) of the PEG hydrogels cross-linked with MMP-sensitive linker B. N = 3.
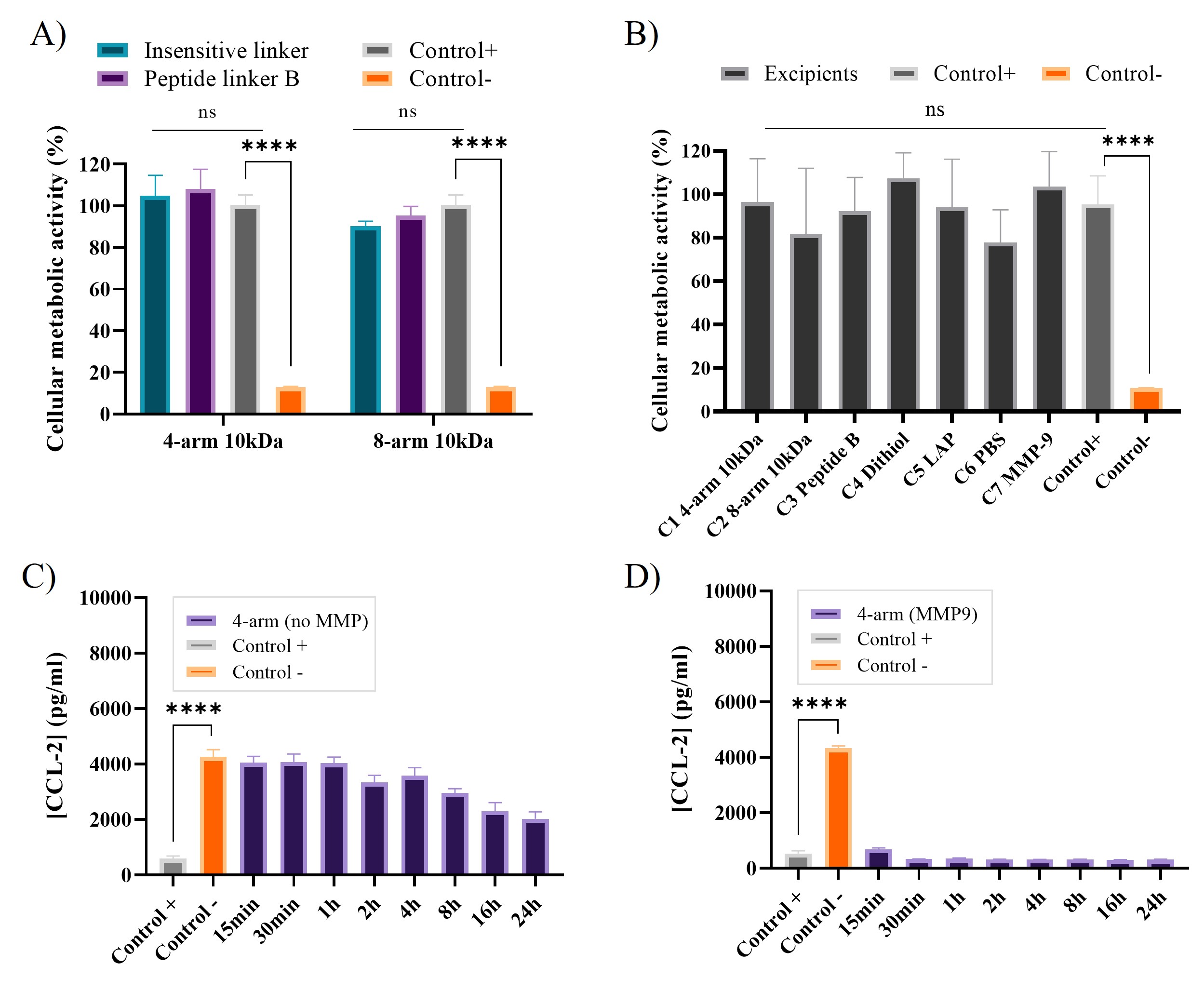
Figure 3. (A) Results obtained from the fibroblast cytotoxicity assay for evaluation of the hydrogel cleavage products after exposure to MMP-9. (B) Corresponding results obtained for separate formulation components. The incubation time for (A - B) was 24 h. Results from the AD-like keratinocyte assay showing the CCL-2 inhibition by tofacitinib citrate at different timeslots. (C) 4-arm hydrogels without MMP-9 stimulus, (D) 4-arm hydrogels exposed to 20 nM MMP-9 stimulus. N = 3.
Formulation and Delivery - Chemical - Drug Delivery
Category: Late Breaking Poster Abstract
(W1230-01-02) Matrix Metalloproteinase-Responsive Hydrogels for the Treatment of Inflammatory Skin Diseases
Wednesday, October 19, 2022
12:30 PM – 1:30 PM ET
- HN
Heidi Noddeland, MA
LEO Pharma A/S
Ballerup, Hovedstaden, Denmark - HN
Heidi Noddeland, MA
LEO Pharma A/S
Ballerup, Hovedstaden, Denmark
Presenting Author(s)
Main Author(s)
Purpose: Designing novel drug delivery systems is key to enable new pharmaceutical products to improve patient health by enhancing the delivery of a therapeutic to its target, improving its safety, and facilitating patient compliance [1, 2]. The development of new “intelligent” materials has advanced the field of emerging technologies such as enzyme-responsive drug delivery systems [3, 4]. In particular, matrix metalloproteinase (MMP) responsive hydrogels represent promising opportunities for intradermal on-demand delivery due to the hydrated network where enzymes can come in close proximity and interact with the sensitive material or the incorporated sensitive moiety [5-9]. Here, we report MMP-responsive hydrogels formed by step-growth photopolymerization of biscysteine peptide linkers with norbornene functionalized branched polyethylene glycols for the treatment of inflammatory skin diseases. To validate the MMP-responsive hydrogels for their intended use they were loaded with a Janus kinase (JAK) inhibitor tofacitinib citrate. The MMP responsiveness and the biocompatibility of the hydrogels were evaluated, and the bioactivity of the released tofacitinib citrate was investigated in an atopic dermatitis (AD) like keratinocyte assay.
Methods: 1H high resolution magic angle spinning nuclear magnetic resonance (HR-MAS NMR) spectroscopy (Bruker Avance 600 HD III, 14.1 T) was used to investigate the conversion of norbornenes and thiols to thioethers via the disappearance alkene protons peaks of norbornene under hydrated conditions. In vitro release studies were performed using Franz Diffusion cells with a nitrocellulose membrane (molecular cut-off: 9 kDa). Tofacitinib citrate was quantified using HPLC (Waters ACQUITY UPLC H-class system, Massachusetts, US). The biocompatibility study was conducted with fibroblasts (murine 3T3) and the anti-inflammatory activity of tofacitinib citrate released from the hydrogels was verified employing human epidermal keratinocytes which was stimulated with a cocktail of T-cell cytokines characteristic of the inflammatory skin disease AD (IL-4, IL-13, IL-22 and INF-γ) [10].
Results: 1H HR-MAS NMR enabled quantitative investigation of the degree of cross-linking in hydrated multi-arm PEG hydrogels after photopolymerization. Firstly, we found that the photo-initiator LAP was more efficient than Irgacure 2959, resulting in a ~100% disappearance of the C=C double bond in the multi-arm PEG functionalized NB groups within 30 s (Figure 1A). Secondly, we noted that the degree of cross-linking changed remarkably with the PEG polymer concentration (Figure 1B). Irrespective of the cross-linker, almost complete cross-linking was seen for hydrogels with polymer concentrations of 5% and 10% (w/w) of both 4-arm and 8-arm PEG hydrogels, respectively. In the absence of MMP stimulus, a cumulative release of 32% and 25% of the total amount of tofacitinib citrate in the hydrogels was seen after 48 h incubation for 4-arm and 8-arm MMP-responsive PEG hydrogels, respectively. Upon addition of 20 nM MMP-9, the cumulative release of tofacitinib citrate reached 67% and 49% for 4-arm and 8-arm PEG hydrogels, respectively (Figure 2A). As a control, the non-responsive 4-arm and 8-arm PEG hydrogels were tested in parallel, and no significant difference in the cumulative release was found between MMP-9-exposed hydrogels and controls (Figure 2B). The cumulative release observed in the absence of MMP-9 was lower for the 8-arm than for 4-arm hydrogels cross-linked (Figure 2C-D). To mimic the flare-up characteristics seen in several inflammatory skin diseases and to investigate the enzymatic release associated with such flare-ups, activated MMP-9 was repeatedly. Both 4-arm and 8-arm PEG hydrogels were found to respond to the addition of newly activated MMP-9 stimulus, as confirmed by the stepwise increase in cumulative release (Figure 2E). Next, we investigated the cytotoxicity of hydrogel breakdown products after exposure to MMP-9 (Figure 3A), as well as to all separate hydrogel components employing 3T3 fibroblasts (Figure 3B). No significant reduction in cell viability was observed for either the MMP-9 generated cleavage products or the separate hydrogel components. In the AD keratinocyte assay it was found that samples collected from experiments without MMP-9 do not inhibit the production of CCL-2 in the early sampling times ( < 1 h) for 4-arm PEG (Figure 3C). When the hydrogels were exposed to 20nM MMP-9, however, the resulting increased tofacitinib citrate concentration present after triggered release was found to result in full inhibition of CCL-2 production at all sampling times for 4-arm PEG hydrogels (Figure 3D).
Conclusion: Here, we have presented a rational design of MMP-responsive hydrogels formed by thiol-norbornene photopolymerization for the treatment of inflammatory skin disease. As a pre-clinical proof-of-concept, we verified that such hydrogels can be designed to deliver tofacitinib citrate in response to MMP-9 exposure. Under the conditions investigated, no cytotoxicity was observed, neither related to the MMP-responsive hydrogels nor to the cleavage products generated during their exposure to MMP-9. In an AD-like keratinocyte assay, we further confirmed that the incorporation of tofacitinib citrate into the hydrogels does not compromise the anti-inflammatory effect of tofacitinib citrate. Translation into an intradermal depot of such hydrogels seems to be promising with respect to the treatment of flare-up driven diseases characterized by high MMP activity.
References: 1. May, M., Why drug delivery is the key to new medicines. Nat Med, 2022. 21(6): p. 1100-1102.
2. Lu, Y., et al., Bioresponsive materials. Nature Reviews Materials, 2016. 2(1).
3. Fairbanks, B.D., et al., A Versatile Synthetic Extracellular Matrix Mimic via Thiol-Norbornene Photopolymerization. Adv Mater, 2009. 21(48): p. 5005-5010.
4. Shahriari, M., et al., Enzyme responsive drug delivery systems in cancer treatment. J Control Release, 2019. 308: p. 172-189.
5. Sobczak, M., Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems-State of Knowledge and Future Prospects. Int J Mol Sci, 2022. 23(8).
6. Yao, Q., et al., MMP-Responsive 'Smart' Drug Delivery and Tumor Targeting. Trends Pharmacol Sci, 2018. 39(8): p. 766-781.
7. Guo, J.W. and S.H. Jee, Strategies to Develop a Suitable Formulation for Inflammatory Skin Disease Treatment. Int J Mol Sci, 2021. 22(11).
8. Stewart-McGuinness, C., et al., Defining the Protease and Protease Inhibitor (P/PI) Proteomes of Healthy and Diseased Human Skin by Modified Systematic Review. Biomolecules, 2022. 12(3).
9. Eckhard, U., et al., Active site specificity profiling of the matrix metalloproteinase family: Proteomic identification of 4300 cleavage sites by nine MMPs explored with structural and synthetic peptide cleavage analyses. Matrix Biol, 2016. 49: p. 37-60.
10. Tine Skak-Nielsen , N.W.H., Inhibition of CCL2 release from IL-4, IL-13, IL-22 and IFNg-stimulated primary human keratinocytes. LEO Pharma Open Innovation, 2020.
Acknowledgments: The authors acknowledge Dan Buchvardt from Quality Laboratories Denmark, LEO Pharma A/S for the support with the mechanical compression plate testing and Birgitte Karna Davidsen from In Vitro Biology, LEO Pharma A/S for assistance with the cell handling of keratinocytes and fibroblast.
Funding: The work reported in this paper was financially supported by LEO Pharma A/S (2750 Ballerup, Denmark), as well as the LEO Foundation Center for Cutaneous Drug Delivery, University of Copenhagen (Grant number 2016-11-01; AH, MM)
Declaration of interest: The authors declare no conflicts of interest.

Figure 1. (A) Investigation of cross-linking degree in hydrated PEG hydrogels using 1H HR-MAS NMR spectroscopy. 1H NMR spectra for formulations containing Irgacure (0.05%, w/w) and LAP (0.05%, w/w) are shown. The protons originating from the double bond in norbornene can be detected between 5.95 and 6.30 ppm. (B) 1H HR-MAS NMR spectroscopy results on the cross-linking degree for hydrogels prepared using non-responsive linker, peptide linker A, and peptide linker B. The hydrogels were crosslinked by UV light (365 nm) for 30 s. N = 3.

Figure 2. Cumulative release of tofacitinib citrate from 5% (w/w) 4-arm and 8-arm PEG (w/w) hydrogels in PBS at 37 °C without and with 20 nM activated MMP-9, respectively. Results are shown for PEG hydrogels cross-linked with (A) MMP-sensitive linker B and (B) non-responsive linker. The MMP-responsive cumulative drug release is shown after 48 h for hydrogels for (C) MMP-sensitive linker B and (D) MMP-insensitive linker. (E) Cumulative drug release after repeated MMP-9 exposure (↓) of the PEG hydrogels cross-linked with MMP-sensitive linker B. N = 3.

Figure 3. (A) Results obtained from the fibroblast cytotoxicity assay for evaluation of the hydrogel cleavage products after exposure to MMP-9. (B) Corresponding results obtained for separate formulation components. The incubation time for (A - B) was 24 h. Results from the AD-like keratinocyte assay showing the CCL-2 inhibition by tofacitinib citrate at different timeslots. (C) 4-arm hydrogels without MMP-9 stimulus, (D) 4-arm hydrogels exposed to 20 nM MMP-9 stimulus. N = 3.
