Back
Purpose: Oral administration remains the most sought-after method for delivery of drugs owing to ease of administration, cost-effectiveness and compliance. However, the poor stability of peptides and proteins in the GI tract, especially in the low pH of the stomach, makes oral delivery of these molecules extremely challenging. Deficiency of digestive enzymes, especially the pancreatic lipase leads to a condition called exocrine pancreatic insufficiency (EPI). Administration of porcine pancreatic enzyme replacement therapy (PERT) remains the primary treatment option for patients with EPI. Adrulipase, a recombinant non-porcine lipase is currently in development for the treatment of EPI. In the present study, several formulations of Adrulipase as a spray dried dispersion (SDD) were evaluated towards achieving an optimal delayed release profile while retaining adrulipase activity. A stability study of the selected lead SDD was also conducted.
Methods: Spray drying: Aqueous solutions of Adrulipase (API) with different excipients were spray dried using a Buchi B290 mini spray-drier (two fluid nozzle) with an inlet temperature of 90-140°C, air flow rate of 400-700 L/h, aspirator flow rate of 25-40 m3/min and liquid feed rate of 2-6 mL/min. Nine different spray dried dispersions were generated using API, maltodextrin, hydroxypropyl methylcellulose phthalate (HPMCP), hydroxypropyl methylcellulose acetate succinate (HPMCAS) and arginine in different proportions. Transitional Dissolution testing: SDD powder (~75 mg Lipase*) was added to 100 mL of 20mM citrate buffer pH 3.0 at 37°C in an Agilent type II dissolution apparatus. At 30 minutes ~4.5mL of 1N NaOH was added to the vessel to shift the pH to 6.5. 1mL samples were taken at 15, 30, 35, 45, 60, and 90 minutes. Samples from each time point were centrifuged (16,873 RCF for 5 min) and analyzed via the Bradford assay to determine enzyme recovery and release profiles. The samples of each SDD were also evaluated in 20mM Phosphate buffer pH 6.5 as controls. *~30 mg for SDD01 and SDD02. Colorimetric Activity Assay: Choi et.al (2003) reported a UV-Vis based colorimetric plate assay to test lipase activity[1]. This procedure was modified and optimized for the current study. Stability Study: A 12-week stability study of the selected lead SDD (SDD04) was conducted at 40°C/75% RH and 25°C/60% RH. ~50 mg SDD04 was filled in glass vials and kept under respective stability conditions. Tests included HPLC-SEC analysis, activity assay and appearance at 0, 1 week, 2 weeks, 4 weeks, 8 weeks and 12 weeks.
Results: Adrulipase showed similar enzymatic activity before and after spray drying. SDD01-SDD04 contained maltodextrin as the stabilizer with or without HPMCP. SDD06 and SDD07 contained HPMCP with Arginine instead of Maltodextrin. HPMCP was replaced with HPMCAS in SDD08 with Maltodextrin as the stabilizing agent. SDD05 contained spray dried API with no excipients. The transitional dissolution profiles of the SDDs are shown in Figure 1. SDD02 and SDD03 contained no enteric polymers ( >80% release at pH 3). SDD containing arginine (SDD07) showed incomplete release at pH 6.5 versus SDD containing maltodextrin (SDD04) at similar drug load. An attempt was made to maximize drug load by spray drying just API with HPMCP, however the drug release from the SDD was slow and incomplete (SDD09). HPMCP provided better enteric protection than HPMCAS (SDD04 versus SDD08, 0-10% versus ~40% release at pH 3) at similar concentration and drug loading. SDD04 was selected as the lead for further development of capsule dosage form. The % spray dried yield was approx. 65-70%, contained approximately 50% drug load, was fully protected at pH 3, and retained 50-60% activity even after 90 minutes under dissolution conditions. No significant change in HPLC-SEC peak area as well as activity was observed on storage of the material at both stability conditions (Figure 2). Evaluation of powder flow properties revealed poor flow and compressibility. Granulation experiments are currently in progress to improve powder flow and maintain greater enzymatic viability during transitional dissolution.
Conclusion: Nine Adrulipase spray-dried dispersions (SDDs) containing various excipients (HPMCP, HPMCAS, maltodextrin and arginine) were prepared and characterized. With the goal to generate drug product with a delayed release profile and the highest possible Adrulipase loading, formulation SDD04 was selected as the lead formulation for further development. SDD04 showed delayed release profile, offering the best protection at acidic pH and rapid release under intestinal conditions, as well as minor loss of activity over 1) 3-month stability assessment (25°C and 40 °C) and 2) formulation processing activities (granulation). Granulation experiments to improve flowability and better retention of enzymatic activity during dissolution are ongoing and have shown great promise. SDD of enzymes with a stabilizing agent and an enteric polymer may provide a novel approach to enable oral delivery of peptides and proteins as therapeutics.
References: 1. Choi, S. J., Hwang, J. M., & Kim, S. I. (2003). A colorimetric microplate assay method for high throughput analysis of lipase activity. BMB Reports, 36(4), 417-420.
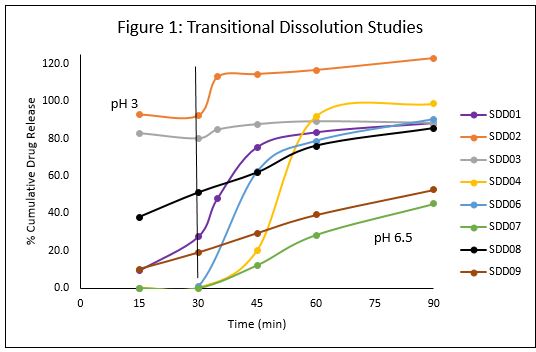
Figure 1: Transitional Dissolution Studies
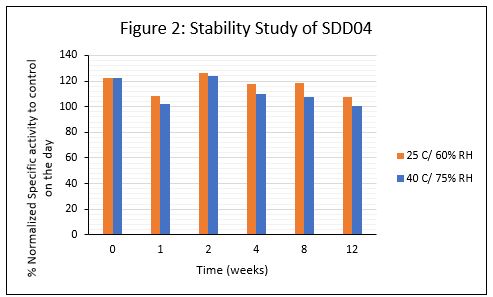
Figure 2: Stability Study of SDD04
Formulation and Delivery - Biomolecular - Formulation
Category: Poster Abstract
(W0930-01-02) Formulation Development of Enterically Protected Spray Dried Dispersions of Adrulipase
Wednesday, October 19, 2022
9:30 AM – 10:30 AM ET
- HD
Heta Desai, Ph.D.
Pace Life Sciences
Melrose, Massachusetts, United States - HD
Heta Desai, Ph.D.
Pace Life Sciences
Melrose, Massachusetts, United States
Presenting Author(s)
Main Author(s)
Purpose: Oral administration remains the most sought-after method for delivery of drugs owing to ease of administration, cost-effectiveness and compliance. However, the poor stability of peptides and proteins in the GI tract, especially in the low pH of the stomach, makes oral delivery of these molecules extremely challenging. Deficiency of digestive enzymes, especially the pancreatic lipase leads to a condition called exocrine pancreatic insufficiency (EPI). Administration of porcine pancreatic enzyme replacement therapy (PERT) remains the primary treatment option for patients with EPI. Adrulipase, a recombinant non-porcine lipase is currently in development for the treatment of EPI. In the present study, several formulations of Adrulipase as a spray dried dispersion (SDD) were evaluated towards achieving an optimal delayed release profile while retaining adrulipase activity. A stability study of the selected lead SDD was also conducted.
Methods: Spray drying: Aqueous solutions of Adrulipase (API) with different excipients were spray dried using a Buchi B290 mini spray-drier (two fluid nozzle) with an inlet temperature of 90-140°C, air flow rate of 400-700 L/h, aspirator flow rate of 25-40 m3/min and liquid feed rate of 2-6 mL/min. Nine different spray dried dispersions were generated using API, maltodextrin, hydroxypropyl methylcellulose phthalate (HPMCP), hydroxypropyl methylcellulose acetate succinate (HPMCAS) and arginine in different proportions. Transitional Dissolution testing: SDD powder (~75 mg Lipase*) was added to 100 mL of 20mM citrate buffer pH 3.0 at 37°C in an Agilent type II dissolution apparatus. At 30 minutes ~4.5mL of 1N NaOH was added to the vessel to shift the pH to 6.5. 1mL samples were taken at 15, 30, 35, 45, 60, and 90 minutes. Samples from each time point were centrifuged (16,873 RCF for 5 min) and analyzed via the Bradford assay to determine enzyme recovery and release profiles. The samples of each SDD were also evaluated in 20mM Phosphate buffer pH 6.5 as controls. *~30 mg for SDD01 and SDD02. Colorimetric Activity Assay: Choi et.al (2003) reported a UV-Vis based colorimetric plate assay to test lipase activity[1]. This procedure was modified and optimized for the current study. Stability Study: A 12-week stability study of the selected lead SDD (SDD04) was conducted at 40°C/75% RH and 25°C/60% RH. ~50 mg SDD04 was filled in glass vials and kept under respective stability conditions. Tests included HPLC-SEC analysis, activity assay and appearance at 0, 1 week, 2 weeks, 4 weeks, 8 weeks and 12 weeks.
Results: Adrulipase showed similar enzymatic activity before and after spray drying. SDD01-SDD04 contained maltodextrin as the stabilizer with or without HPMCP. SDD06 and SDD07 contained HPMCP with Arginine instead of Maltodextrin. HPMCP was replaced with HPMCAS in SDD08 with Maltodextrin as the stabilizing agent. SDD05 contained spray dried API with no excipients. The transitional dissolution profiles of the SDDs are shown in Figure 1. SDD02 and SDD03 contained no enteric polymers ( >80% release at pH 3). SDD containing arginine (SDD07) showed incomplete release at pH 6.5 versus SDD containing maltodextrin (SDD04) at similar drug load. An attempt was made to maximize drug load by spray drying just API with HPMCP, however the drug release from the SDD was slow and incomplete (SDD09). HPMCP provided better enteric protection than HPMCAS (SDD04 versus SDD08, 0-10% versus ~40% release at pH 3) at similar concentration and drug loading. SDD04 was selected as the lead for further development of capsule dosage form. The % spray dried yield was approx. 65-70%, contained approximately 50% drug load, was fully protected at pH 3, and retained 50-60% activity even after 90 minutes under dissolution conditions. No significant change in HPLC-SEC peak area as well as activity was observed on storage of the material at both stability conditions (Figure 2). Evaluation of powder flow properties revealed poor flow and compressibility. Granulation experiments are currently in progress to improve powder flow and maintain greater enzymatic viability during transitional dissolution.
Conclusion: Nine Adrulipase spray-dried dispersions (SDDs) containing various excipients (HPMCP, HPMCAS, maltodextrin and arginine) were prepared and characterized. With the goal to generate drug product with a delayed release profile and the highest possible Adrulipase loading, formulation SDD04 was selected as the lead formulation for further development. SDD04 showed delayed release profile, offering the best protection at acidic pH and rapid release under intestinal conditions, as well as minor loss of activity over 1) 3-month stability assessment (25°C and 40 °C) and 2) formulation processing activities (granulation). Granulation experiments to improve flowability and better retention of enzymatic activity during dissolution are ongoing and have shown great promise. SDD of enzymes with a stabilizing agent and an enteric polymer may provide a novel approach to enable oral delivery of peptides and proteins as therapeutics.
References: 1. Choi, S. J., Hwang, J. M., & Kim, S. I. (2003). A colorimetric microplate assay method for high throughput analysis of lipase activity. BMB Reports, 36(4), 417-420.

Figure 1: Transitional Dissolution Studies

Figure 2: Stability Study of SDD04
