Back
Purpose: Non-small cell lung cancer (NSCLC) is the second most common cancer and accounts for approximately 80 – 85 % of lung cancer cases reported. Conventional chemotherapeutics show limited application because of poor tumor selectivity and acquired drug resistance. Hence, there is a continued need to develop novel anticancer therapies with minimal adverse events and lower chances of acquiring drug resistance. Recently, antimicrobial peptides (AMPs) have gained interest as anticancer agents due to their high potency and high target selectivity with limited scope for drug resistance. Also, AMPs could inhibit cancer cell proliferation via both membranolytic (membrane disruption) and non-membranolytic (unsettling energy metabolism) pathways. Synthetic linear cationic amphipathic α-helical peptide, D-LAK-120A (D-LAK), is cationic, and shows better biostability and antimicrobial activity in vitro. In this study, we evaluated anticancer efficacy and mechanism of action of D-LAK in various NSCLC cell lines.
Methods: The cytotoxicity was evaluated by changes in mitochondrial metabolic activity (reduction of MTT) after 72 h treatment with D-LAK in KRAS-mutated A549 (G12S, p53 protein overexpression) and H358 (G12C, non-functional p53 tumor protein), and EGFR-mutated H1975 (L858R/T790M and Exon 19 deletion) and HCC827 (E746-A750 deletion) lung adenocarcinoma cell lines. Also, we evaluated cell viability in healthy (HEK293) cells. The membranolytic inhibitory pathway was investigated by changes in membrane integrity (lactate dehydrogenase (LDH) assay). Further, the non-membranolytic pathway of cancer cell inhibition was verified by apoptosis assay (Annexin V staining), cell cycle analysis (propidium iodide staining), mitochondrial membrane potential assay (tetramethyl rhodamine ethyl ester; TMRE mitochondria labeling), and reactive oxygen species (ROS) analysis (DCFDA assay). In addition, the effect of D-LAK on clonal expansion and metastatic properties was evaluated by clonogenic and wound healing assay, respectively. Lastly, in vitro tumor simulation studies were performed by forming 3D spheroid of A549 cells in ultra-low attachment microplates.
Results: The D-LAK peptide demonstrated enhanced cytotoxicity in A549, H358, H1975, and HCC827 cell lines with inhibitory concentrations between 4.0 – 5.5 µM (fig. 1a), suggesting inhibition of NSCLC irrespective of their genetic mutations (KRAS and EGFR) and tumor protein (p53) expression. In healthy cells, D-LAK exhibited higher inhibitory concentration (8.0 ± 0.25 µM) than for cancer cells, confirming its safety profile. An increase in the lactate dehydrogenase (LDH) levels suggested the membranolytic activity (pore formation) as an inhibition pathway (fig. 1b). In addition, we found D-LAK induced lung cancer cell apoptosis (fig. 2a) and arrested cells in the S phase (DNA synthesis) of the cell cycle (fig. 2b). Moreover, a decreased mitochondrial membrane potential (referred to as membrane depolarization; fig. 2c) and increased ROS levels (fig. 2d) were observed in A549 cells in a concentration-dependent manner after D-LAK treatment, suggesting induction of mitochondria-mediated apoptosis, a non-membranolytic pathway of cancer cell inhibition. Additionally, D-LAK inhibited single cell proliferation (reduction in colony formation; fig. 3a) and cancer cell migration (a time- and dose-dependent wound closure inhibition; fig. 3b) of advanced NSCLC cells in vitro. In tumor simulation studies, D-LAK (6 µM) treatment group significantly inhibited spheroid growth (0.65-fold change in volume) compared to control (2.5-fold increase in volume) and D-LAK (3 µM; 2.5-fold increase in volume) after 9 days of treatment (fig. 3d). The spheroid cell viability for D-LAK was reduced to 22.3 ± 1.8 % compared to control group at the end of the treatment period (as analyzed by luminescence assay; fig. 3c). A similar trend was observed in the live-dead assay where dead cells (red, EthD-III stained cells) are in the periphery of the live spheroid (stained by green, Calcein AM dye) in 6 mM treatment group (fig. 3e), thereby strengthening the case for the efficacy of D-LAK for NSCLC treatment in a multiple-dose regimen.
Conclusion: D-LAK resulted in LDH release, cell cycle arrest, elevation in ROS levels, and mitochondria-mediated apoptosis suggesting inhibition of NSCLC by both membranolytic and non-membranolytic pathways. Furthermore, inhibition of metastatic characteristics and reduction in spheroid growth shows the enhanced anticancer activity of D-LAK against NSCLC in vitro. These results obtained in this study provide preliminary evidence for applying D-LAK as an anticancer agent in a preclinical setup.
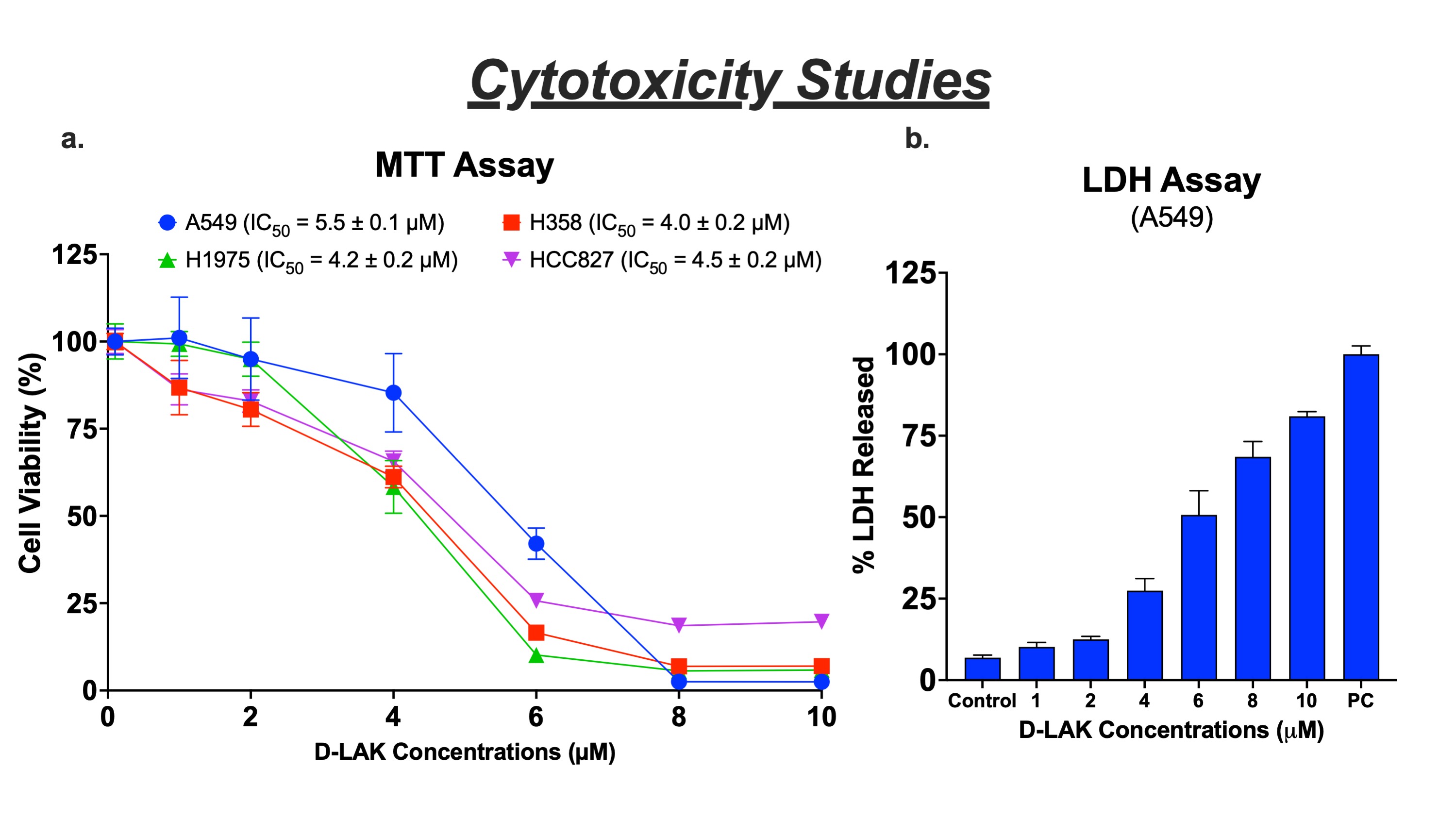
Figure 1. Cytotoxicity studies of D-LAK in NSCLC cell lines. a. Cell viability studies after 72 h treatment with D-LAK determined using MTT assay in A549, H358, H1975, and HCC827 NSCLC cell lines. b. LDH was released from A549 cells following treatment with D-LAK. (Mean ± SD, n=3).
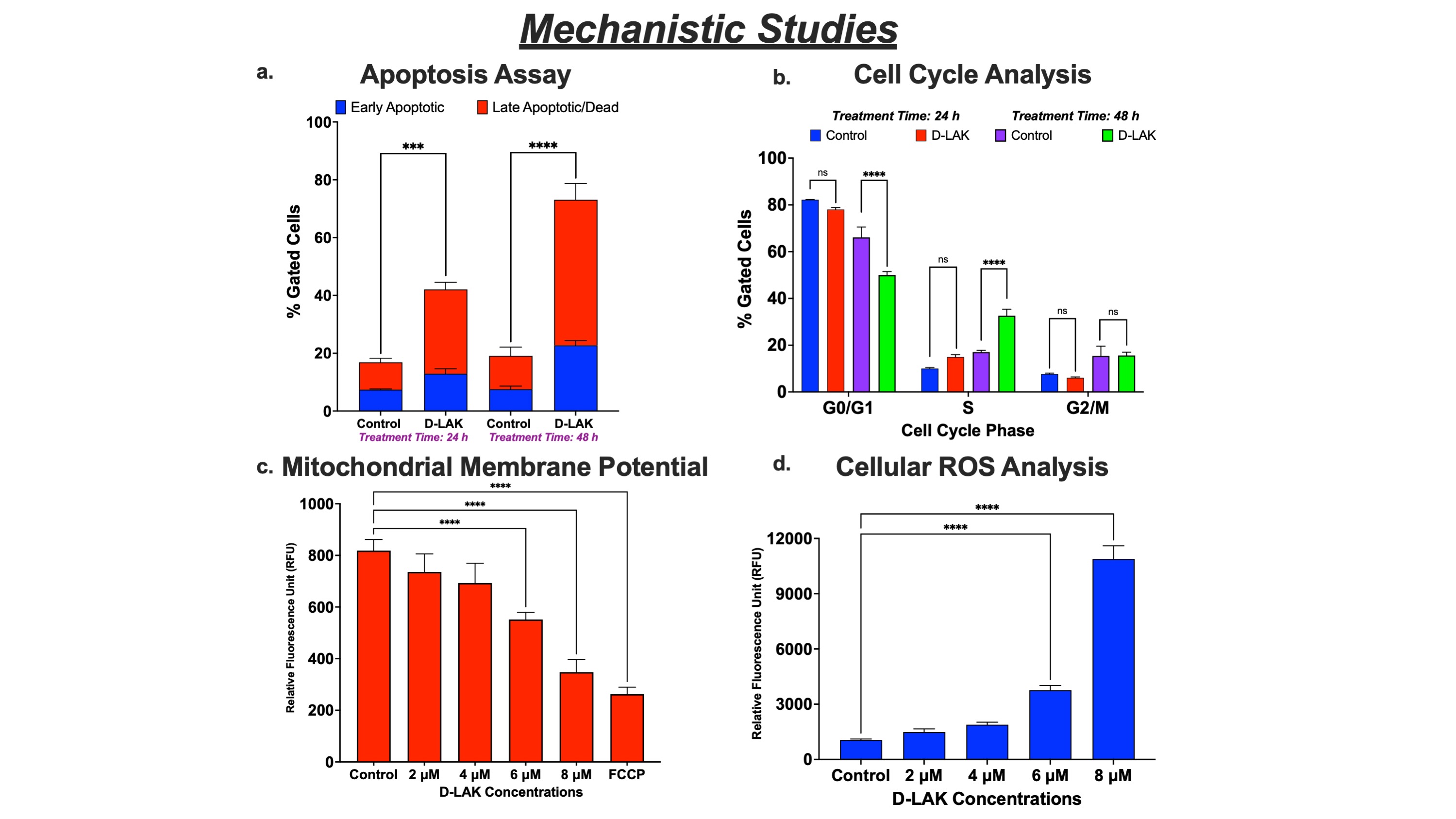
Figure 2. Mechanistic Studies of D-LAK in A549 cells. a. Early apoptotic and late apoptotic/dead cells quantified by Annexin V Assay demonstrate time-dependent induction of apoptosis. b. Quantification of cells based on cell cycle phase showing accumulation in S phase. Influence of energy metabolism on cancer cell inhibition evaluated by c. Mitochondrial membrane potential analysis (reduction in potential suggesting membrane depolarization) and d. ROS Assay (increase in ROS levels) after treatment with different D-LAK concentrations.
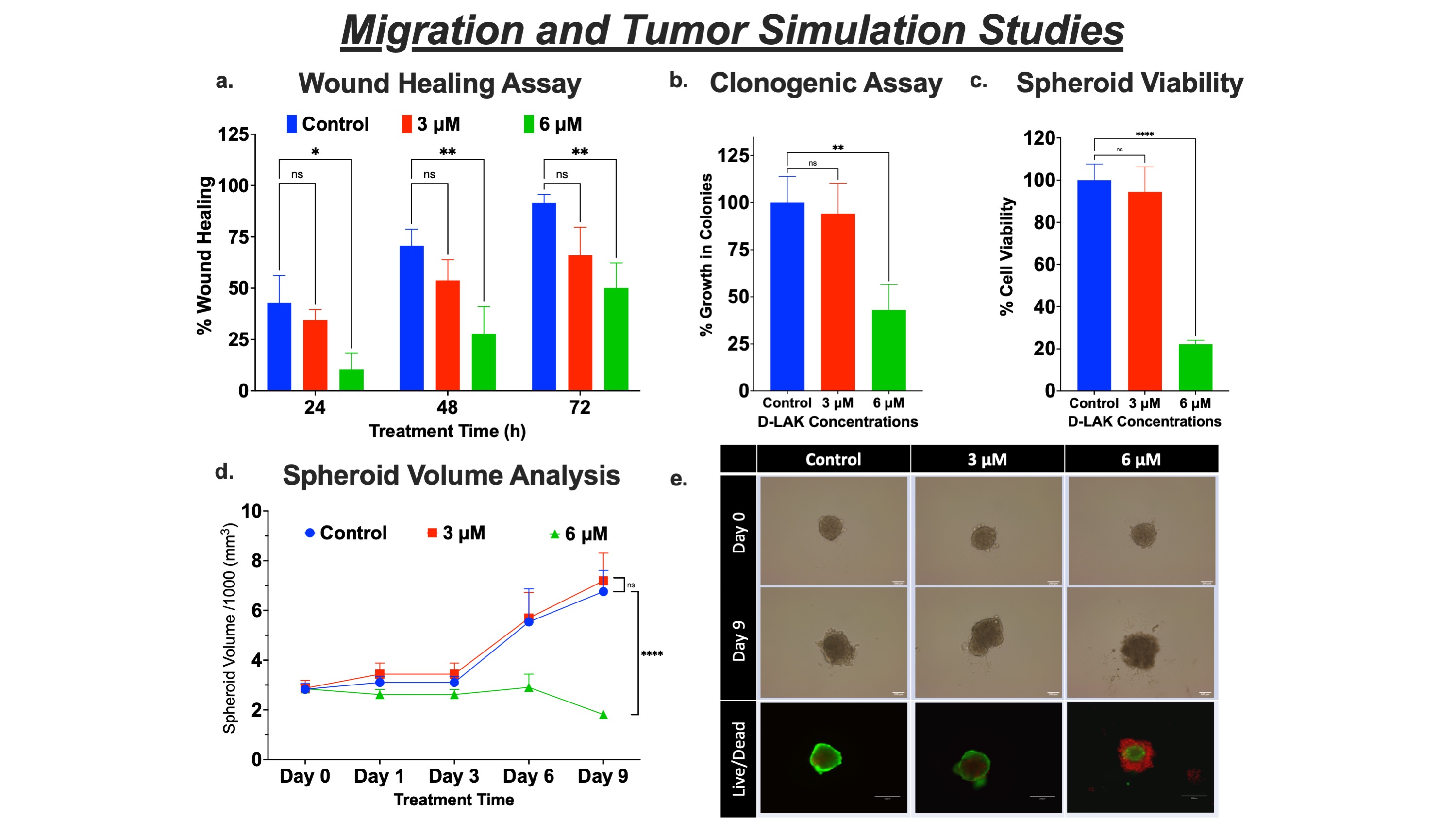
Figure 3. Migration and Tumor Simulation Studies in A549 cells. a. Wound healing assay analysis of A549 cells, shown as % wound healing over time, after treatment with D-LAK. b. Clonogenic assay analysis after 72 h of D-LAK treatment. c. Cell viability assay of 3D spheroids obtained using Cell-titer Glo assay. d. Spheroid growth curve (change in tumor volume over the course of treatment; n = 6). e. Illustrative images of spheroids after multiple doses at days 0 and 9 and fluorescent images of spheroid after live-dead cell assay.
Discovery and Basic Research - Medicinal Chemistry
Category: Poster Abstract
(M1330-05-26) The Cationic Antimicrobial Peptide, D-LAK-120A, as a Potential Anti-cancer Therapeutic for Non-small Cell Lung Cancer (NSCLC)
Monday, October 17, 2022
1:30 PM – 2:30 PM ET
- SP
Suyash M. Patil
St. John's University
Queens, New York, United States - SP
Suyash M. Patil
St. John's University
Queens, New York, United States
Presenting Author(s)
Main Author(s)
Purpose: Non-small cell lung cancer (NSCLC) is the second most common cancer and accounts for approximately 80 – 85 % of lung cancer cases reported. Conventional chemotherapeutics show limited application because of poor tumor selectivity and acquired drug resistance. Hence, there is a continued need to develop novel anticancer therapies with minimal adverse events and lower chances of acquiring drug resistance. Recently, antimicrobial peptides (AMPs) have gained interest as anticancer agents due to their high potency and high target selectivity with limited scope for drug resistance. Also, AMPs could inhibit cancer cell proliferation via both membranolytic (membrane disruption) and non-membranolytic (unsettling energy metabolism) pathways. Synthetic linear cationic amphipathic α-helical peptide, D-LAK-120A (D-LAK), is cationic, and shows better biostability and antimicrobial activity in vitro. In this study, we evaluated anticancer efficacy and mechanism of action of D-LAK in various NSCLC cell lines.
Methods: The cytotoxicity was evaluated by changes in mitochondrial metabolic activity (reduction of MTT) after 72 h treatment with D-LAK in KRAS-mutated A549 (G12S, p53 protein overexpression) and H358 (G12C, non-functional p53 tumor protein), and EGFR-mutated H1975 (L858R/T790M and Exon 19 deletion) and HCC827 (E746-A750 deletion) lung adenocarcinoma cell lines. Also, we evaluated cell viability in healthy (HEK293) cells. The membranolytic inhibitory pathway was investigated by changes in membrane integrity (lactate dehydrogenase (LDH) assay). Further, the non-membranolytic pathway of cancer cell inhibition was verified by apoptosis assay (Annexin V staining), cell cycle analysis (propidium iodide staining), mitochondrial membrane potential assay (tetramethyl rhodamine ethyl ester; TMRE mitochondria labeling), and reactive oxygen species (ROS) analysis (DCFDA assay). In addition, the effect of D-LAK on clonal expansion and metastatic properties was evaluated by clonogenic and wound healing assay, respectively. Lastly, in vitro tumor simulation studies were performed by forming 3D spheroid of A549 cells in ultra-low attachment microplates.
Results: The D-LAK peptide demonstrated enhanced cytotoxicity in A549, H358, H1975, and HCC827 cell lines with inhibitory concentrations between 4.0 – 5.5 µM (fig. 1a), suggesting inhibition of NSCLC irrespective of their genetic mutations (KRAS and EGFR) and tumor protein (p53) expression. In healthy cells, D-LAK exhibited higher inhibitory concentration (8.0 ± 0.25 µM) than for cancer cells, confirming its safety profile. An increase in the lactate dehydrogenase (LDH) levels suggested the membranolytic activity (pore formation) as an inhibition pathway (fig. 1b). In addition, we found D-LAK induced lung cancer cell apoptosis (fig. 2a) and arrested cells in the S phase (DNA synthesis) of the cell cycle (fig. 2b). Moreover, a decreased mitochondrial membrane potential (referred to as membrane depolarization; fig. 2c) and increased ROS levels (fig. 2d) were observed in A549 cells in a concentration-dependent manner after D-LAK treatment, suggesting induction of mitochondria-mediated apoptosis, a non-membranolytic pathway of cancer cell inhibition. Additionally, D-LAK inhibited single cell proliferation (reduction in colony formation; fig. 3a) and cancer cell migration (a time- and dose-dependent wound closure inhibition; fig. 3b) of advanced NSCLC cells in vitro. In tumor simulation studies, D-LAK (6 µM) treatment group significantly inhibited spheroid growth (0.65-fold change in volume) compared to control (2.5-fold increase in volume) and D-LAK (3 µM; 2.5-fold increase in volume) after 9 days of treatment (fig. 3d). The spheroid cell viability for D-LAK was reduced to 22.3 ± 1.8 % compared to control group at the end of the treatment period (as analyzed by luminescence assay; fig. 3c). A similar trend was observed in the live-dead assay where dead cells (red, EthD-III stained cells) are in the periphery of the live spheroid (stained by green, Calcein AM dye) in 6 mM treatment group (fig. 3e), thereby strengthening the case for the efficacy of D-LAK for NSCLC treatment in a multiple-dose regimen.
Conclusion: D-LAK resulted in LDH release, cell cycle arrest, elevation in ROS levels, and mitochondria-mediated apoptosis suggesting inhibition of NSCLC by both membranolytic and non-membranolytic pathways. Furthermore, inhibition of metastatic characteristics and reduction in spheroid growth shows the enhanced anticancer activity of D-LAK against NSCLC in vitro. These results obtained in this study provide preliminary evidence for applying D-LAK as an anticancer agent in a preclinical setup.

Figure 1. Cytotoxicity studies of D-LAK in NSCLC cell lines. a. Cell viability studies after 72 h treatment with D-LAK determined using MTT assay in A549, H358, H1975, and HCC827 NSCLC cell lines. b. LDH was released from A549 cells following treatment with D-LAK. (Mean ± SD, n=3).

Figure 2. Mechanistic Studies of D-LAK in A549 cells. a. Early apoptotic and late apoptotic/dead cells quantified by Annexin V Assay demonstrate time-dependent induction of apoptosis. b. Quantification of cells based on cell cycle phase showing accumulation in S phase. Influence of energy metabolism on cancer cell inhibition evaluated by c. Mitochondrial membrane potential analysis (reduction in potential suggesting membrane depolarization) and d. ROS Assay (increase in ROS levels) after treatment with different D-LAK concentrations.

Figure 3. Migration and Tumor Simulation Studies in A549 cells. a. Wound healing assay analysis of A549 cells, shown as % wound healing over time, after treatment with D-LAK. b. Clonogenic assay analysis after 72 h of D-LAK treatment. c. Cell viability assay of 3D spheroids obtained using Cell-titer Glo assay. d. Spheroid growth curve (change in tumor volume over the course of treatment; n = 6). e. Illustrative images of spheroids after multiple doses at days 0 and 9 and fluorescent images of spheroid after live-dead cell assay.
