Back
Purpose: The purpose of the study was to develop a series of oral solid modified release (MR) formulations with various release profiles of a novel therapeutic (SCTL-007, which has moderate to high water solubility) to overcome gastrointestinal irritation observed with the immediate release formulations at high doses.
Methods: A series of matrix tablets incorporating 20-30% w/w of Methocel K100M, Methocel K4M or Methocel 100LV were manufactured using direct compression on a single station Natoli RD10 tablet press. The MR tablet assay samples were analyzed on a Waters Acquity UPLC HSS T3 analytical column using gradient elution with a mobile phase consisting of 0.05% trifluoroacetic acid in water and 0.05% trifluoroacetic acid in acetonitrile with UV detection at 248 nm. Standards and samples were diluted with an 80:20 (v/v) mixture of water and acetonitrile to approximately 0.2 mg/mL SCTL-007. In vitro dissolution experiments were performed using USP Apparatus 2 (Paddles) with Japanese Pharmacopeia Basket Sinkers at 75 rpm with 500 mL of 0.1N HCl at 37 ± 0.5°C. Dissolution profiles were generated by sampling at 15 min, 30 min, 45 min, 1 hour, 2 hours, 4 hours, 6 hours, 8 hours, 10 hours, 12 hours, and 14 hours. Additionally, time points at 15 hours and infinity (250 rpm for 30 minutes) were also generated. Samples were filtered through 1 µm inline filters using an autosampler into UPLC vials for analysis. Sample analysis was performed using a Waters H class UPLC equipped with a PDA detector at 248 nm. Isocratic elution using 80% mobile phase A and 20% mobile phase B, where 0.05% trifluoroacetic acid in water as mobile phase A and 0.05% trifluoroacetic acid in methanol as mobile phase B were used. Standards were prepared in 0.01 N HCl to approximately 0.2 mg/mL SCTL-007. A single oral dose of the IR and MR tablets (slowest release-30% Methocel K100M and fastest release-20% Methocel 100LV) were dosed to fasted non-naïve male Beagle dogs (n=5 per treatment), which were pre-treated with 6 µg/kg pentagastrin to normalize stomach pH, and the pharmacokinetic (PK) parameters were determined and compared. The PK blood samples were collected: pre-dose; 0.5, 1, 2, 4, 8, 12, 24, 48, 72, 96, and 120 hr post-dose.
Results: The dissolution method was optimized by evaluating the paddle speed (USP Apparatus II, 50, 75, and 100 rpm) as well as various sinkers (no sinker, custom clip sinkers, and Japanese Pharmacopeia Basket Sinkers). The release of SCTL-007 was similar at 75 and 100 rpm for all formulations evaluated. The use of sinkers was required as the tablets were observed to variable floating and “bouncing” movement during the dissolution which led to high variability in the dissolution results. Incorporation of Japanese Pharmacopeia Basket Sinkers in the dissolution allowed for the tablets to remain at the bottom of the dissolution vessels as well as allowing ample area for the matrix tablets to swell during the dissolution evaluation while affording reproducible results. The release (Q >80%) of SCTL-007 prolonged from 10 min for the IR formulation to 2.5 hr, 6 hr, 11 hr, and 16 hr for the MR formulations by increasing HPMC viscosity and concentration. Further, the dissolution of the MR tablets was independent of the tablet hardness. Only the dogs treated with the IR tablets experienced emesis within 1 hr of dosing, which was not observed for any of the dogs treated with MR tablets. The PK parameters of interest for the IR, 20% Methocel 100LV and 30% Methocel K100M formulations were Cmax: 6840, 9810, and 2690 ng/mL; and AUC0-t: 42600, 72100, and 17200 hr*ng/mL, respectively.
Conclusion: A series of MR formulations with different release profiles were manufactured. The PK results are inconclusive, likely due to the emesis in the IR control group leading to incomplete absorption of the full SCTL-007 dose. However, the MR tablets showed promise in eliminating the gastrointestinal irritation, including emesis, which was the most common drug-related TEAE (treatment emergent adverse event) observed in both the preclinical and clinical studies. Additional PK studies with fasted vs. fed dogs with the existing formulation is recommended.
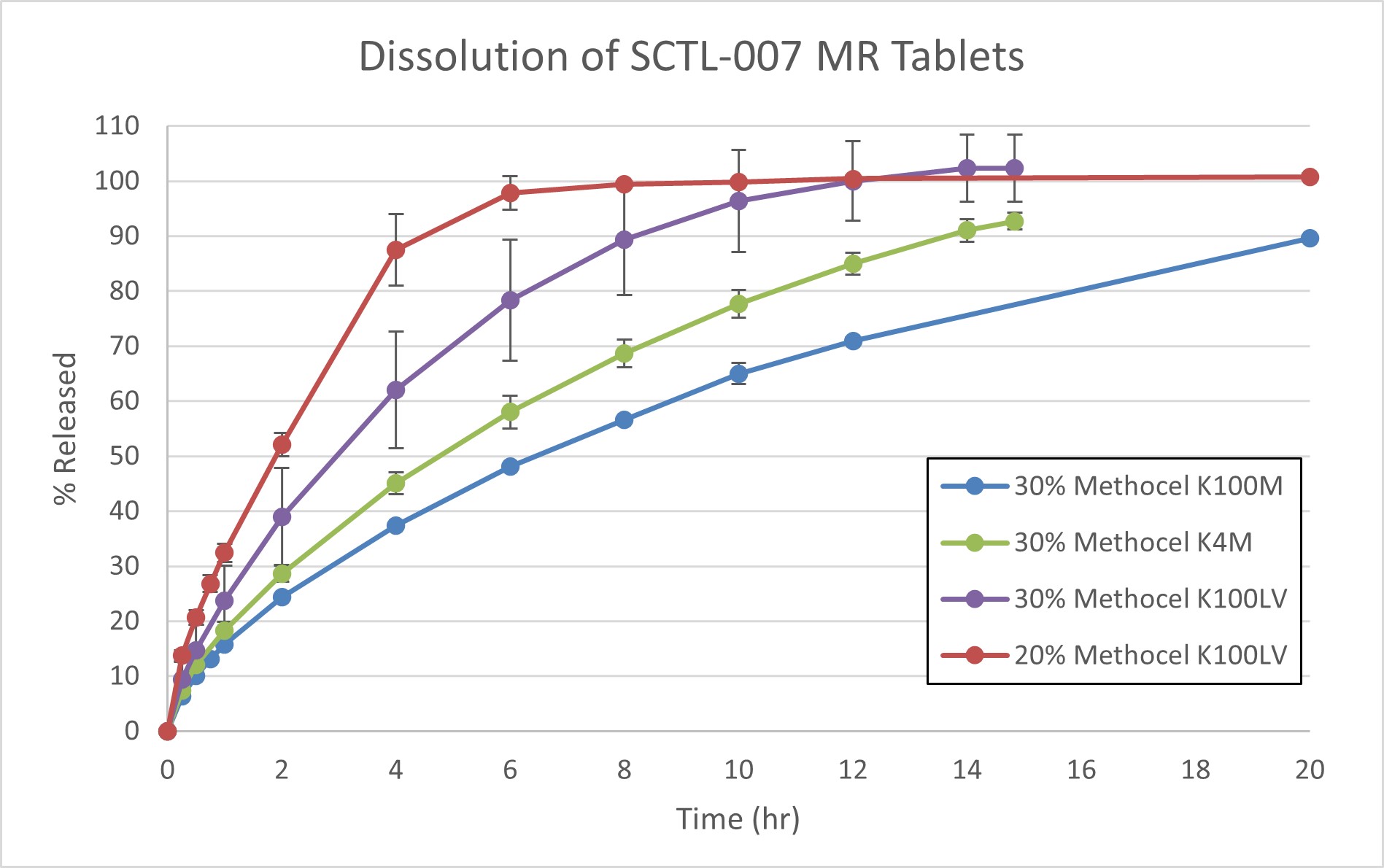
In vitro dissolution of the MR tablet formulations (n=3) was performed using USP Apparatus 2 (Paddles) with Japanese Pharmacopeia Basket Sinkers at 75 rpm with 500 mL of 0.1N HCl at 37 ± 0.5°C.
Formulation and Delivery - Chemical - Formulation
Category: Late Breaking Poster Abstract
(T1130-01-06) Eliminating Upper Gastrointestinal Irritation of a Novel Therapeutic (SCTL-007) by Developing Modified Release Matrix Tablets Utilizing HPMC Polymers
Tuesday, October 18, 2022
11:30 AM – 12:30 PM ET
- TB
Taryn R. Bagby, PhD
Societal CDMO
Gainesville, Georgia, United States - TB
Taryn R. Bagby, PhD
Societal CDMO
Gainesville, Georgia, United States
Presenting Author(s)
Main Author(s)
Purpose: The purpose of the study was to develop a series of oral solid modified release (MR) formulations with various release profiles of a novel therapeutic (SCTL-007, which has moderate to high water solubility) to overcome gastrointestinal irritation observed with the immediate release formulations at high doses.
Methods: A series of matrix tablets incorporating 20-30% w/w of Methocel K100M, Methocel K4M or Methocel 100LV were manufactured using direct compression on a single station Natoli RD10 tablet press. The MR tablet assay samples were analyzed on a Waters Acquity UPLC HSS T3 analytical column using gradient elution with a mobile phase consisting of 0.05% trifluoroacetic acid in water and 0.05% trifluoroacetic acid in acetonitrile with UV detection at 248 nm. Standards and samples were diluted with an 80:20 (v/v) mixture of water and acetonitrile to approximately 0.2 mg/mL SCTL-007. In vitro dissolution experiments were performed using USP Apparatus 2 (Paddles) with Japanese Pharmacopeia Basket Sinkers at 75 rpm with 500 mL of 0.1N HCl at 37 ± 0.5°C. Dissolution profiles were generated by sampling at 15 min, 30 min, 45 min, 1 hour, 2 hours, 4 hours, 6 hours, 8 hours, 10 hours, 12 hours, and 14 hours. Additionally, time points at 15 hours and infinity (250 rpm for 30 minutes) were also generated. Samples were filtered through 1 µm inline filters using an autosampler into UPLC vials for analysis. Sample analysis was performed using a Waters H class UPLC equipped with a PDA detector at 248 nm. Isocratic elution using 80% mobile phase A and 20% mobile phase B, where 0.05% trifluoroacetic acid in water as mobile phase A and 0.05% trifluoroacetic acid in methanol as mobile phase B were used. Standards were prepared in 0.01 N HCl to approximately 0.2 mg/mL SCTL-007. A single oral dose of the IR and MR tablets (slowest release-30% Methocel K100M and fastest release-20% Methocel 100LV) were dosed to fasted non-naïve male Beagle dogs (n=5 per treatment), which were pre-treated with 6 µg/kg pentagastrin to normalize stomach pH, and the pharmacokinetic (PK) parameters were determined and compared. The PK blood samples were collected: pre-dose; 0.5, 1, 2, 4, 8, 12, 24, 48, 72, 96, and 120 hr post-dose.
Results: The dissolution method was optimized by evaluating the paddle speed (USP Apparatus II, 50, 75, and 100 rpm) as well as various sinkers (no sinker, custom clip sinkers, and Japanese Pharmacopeia Basket Sinkers). The release of SCTL-007 was similar at 75 and 100 rpm for all formulations evaluated. The use of sinkers was required as the tablets were observed to variable floating and “bouncing” movement during the dissolution which led to high variability in the dissolution results. Incorporation of Japanese Pharmacopeia Basket Sinkers in the dissolution allowed for the tablets to remain at the bottom of the dissolution vessels as well as allowing ample area for the matrix tablets to swell during the dissolution evaluation while affording reproducible results. The release (Q >80%) of SCTL-007 prolonged from 10 min for the IR formulation to 2.5 hr, 6 hr, 11 hr, and 16 hr for the MR formulations by increasing HPMC viscosity and concentration. Further, the dissolution of the MR tablets was independent of the tablet hardness. Only the dogs treated with the IR tablets experienced emesis within 1 hr of dosing, which was not observed for any of the dogs treated with MR tablets. The PK parameters of interest for the IR, 20% Methocel 100LV and 30% Methocel K100M formulations were Cmax: 6840, 9810, and 2690 ng/mL; and AUC0-t: 42600, 72100, and 17200 hr*ng/mL, respectively.
Conclusion: A series of MR formulations with different release profiles were manufactured. The PK results are inconclusive, likely due to the emesis in the IR control group leading to incomplete absorption of the full SCTL-007 dose. However, the MR tablets showed promise in eliminating the gastrointestinal irritation, including emesis, which was the most common drug-related TEAE (treatment emergent adverse event) observed in both the preclinical and clinical studies. Additional PK studies with fasted vs. fed dogs with the existing formulation is recommended.

In vitro dissolution of the MR tablet formulations (n=3) was performed using USP Apparatus 2 (Paddles) with Japanese Pharmacopeia Basket Sinkers at 75 rpm with 500 mL of 0.1N HCl at 37 ± 0.5°C.
