Back
Purpose: Enteric coatings are designed to protect active pharmaceutical ingredients (APIs) against untimely release in the stomach. It is commonly agreed that acid protection of such coatings depends on the coating layer thickness and integrity, which must be determined in an accurate and reliable way to ensure the desired final product’s performance. Optical coherence tomography (OCT) is an in-line capable technology that allows fast and non-destructive analysis of coating thickness and further material attributes via high-resolution cross-sectional images. A large set of data comprising the coating layer thickness and its uniformity, equally for single tablets (intra-sample coating variability) as well as between tablets (inter-sample coating variability), and the corresponding distributions, can be derived from OCT data. The merits of this technology to link derived coating material attributes to the respective acid protection function has been evaluated in the present study.
Methods: A classical pan coating process was used to apply an enteric coating based on a methacrylic copolymer aqueous dispersion. Three tablet batches (batch A, B and C) were investigated within this study. For each batch, five sample sets consisting of about 100 tablets each were drawn at selected coating time points during the run time of 240 min. An industrial OCT system equipped with a 1D-OCT probe (OSeeT Pharma 1D, Phyllon, Austria) combined with a spinning disc (Schuett-biotec, Germany) setup was used for off-line coating layer analyses. Multiple OCT images of the convex surfaces and the tablet bands of a large number of tablets (90 tablets) per coating time point were acquired for each batch to establish the coating thickness distribution of a representative number of tablets prior to testing their acid stage dissolution performance. In parallel, OCT measurements were performed on additional 6 tablets per coating time point for each batch before the USP dissolution testing of exact these tablets in order to facilitate direct comparison. OCT data and corresponding dissolution data of both samples sets (6 tablets and 90 tablets) were compared to assess their capability and relevance in order to predict a universal acid protection function of the investigated coating.
Results: The acquired data (OCT and dissolution) of the larger sample sets (90 tablets), enabled the calculation of a critical coating thickness required to protect the API from release in the stomach at given confidence interval. In particular, the coating time point exposing sufficient resistance to the gastric fluid was used (150 min, Figure 1), and derived functions used to calculate the critical coating thicknesses of the distribution that lie within a 3 sigma criterion. Accordingly, a critical coating thickness of 27.4 µm for a 3σ criterion could be determined. This information on the critical coating thickness would not have been accessible via the measured mean coating thickness and a standard deviation of 45.5 ± 7.9 µm taken from the corresponding small sample set (6 tablets, 150 min). The reason for this deviation is located in the inclined tailing of the distributions, showing a considerable number of thicknesses below the critical coating thickness for the distributions at earlier stages (i.e., 110 min, where only 80% of the tablets passed, Figure 2). Furthermore, in addition to existing functions used, a new distribution function could be proposed for improved description of the coating thickness distributions in the early stages of industrial coating processes.
Conclusion: It could be demonstrated that the use of OCT enables valuable insights into coating processes, which can be correlated with critical aspects of the drug performance (acid protection). The capabilities of OCT to provide substantial amounts of data on the applied coating thickness, including intra- and inter-sample coating variability and the coating thickness distribution, within seconds enable a more accurate acid protection function prediction. In a nutshell, the presented approach facilitates the calculation of a universal protection function for the investigated coating based on a sound statistical basis originating from the high amount of underlying OCT data. The proposed approach may be further transferred to in-line monitoring of the tablet coating processes, which could drastically improve the production efficiency by ultimately allowing real-time release testing (RTRT).
Acknowledgements: The Research Center Pharmaceutical Engineering (RCPE) is funded within the framework of COMET - Competence Centers for Excellent Technologies by BMK, BMDW, Land Steiermark and SFG. The COMET program is managed by the FFG.
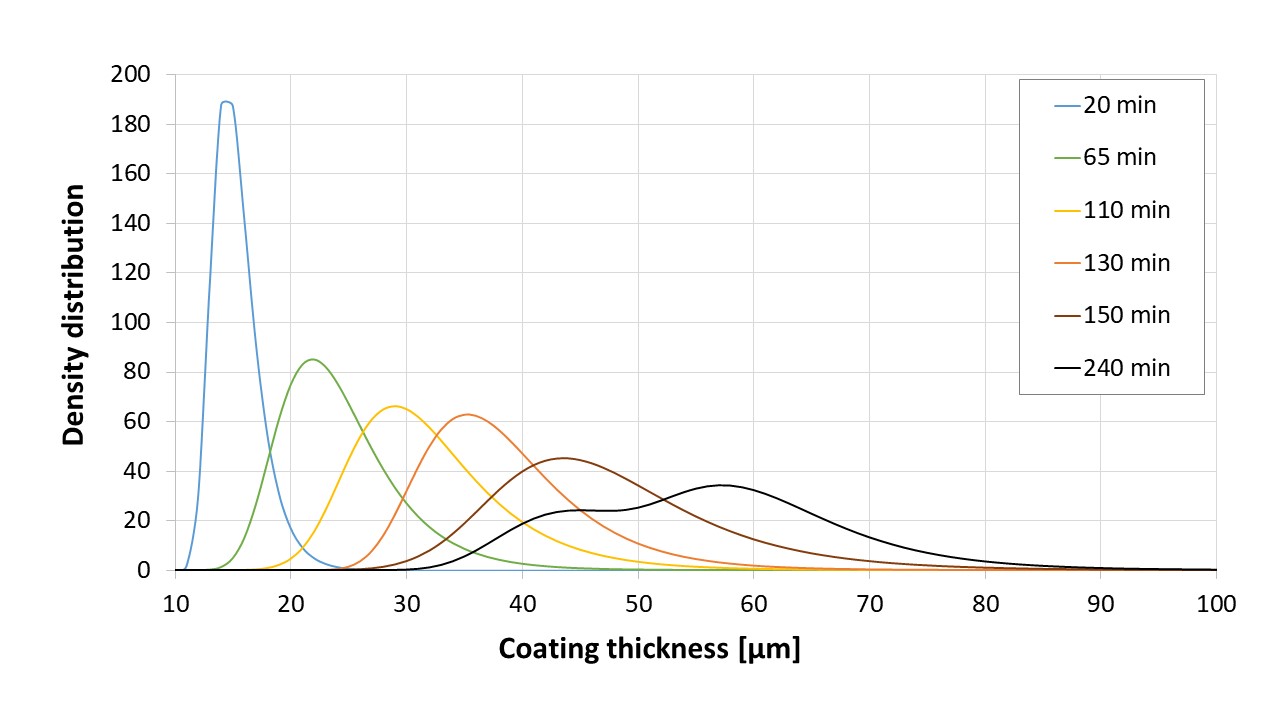
Figure 1: Propagation of coating thickness distributions (mean values of convex surfaces and tablet bands) for the large samples (90 tablets, batch A). Different coating time points are indicated by different colors, the x-axis starts at 10 microns due to the limit of quantification of 12 µm for the OCT system used.
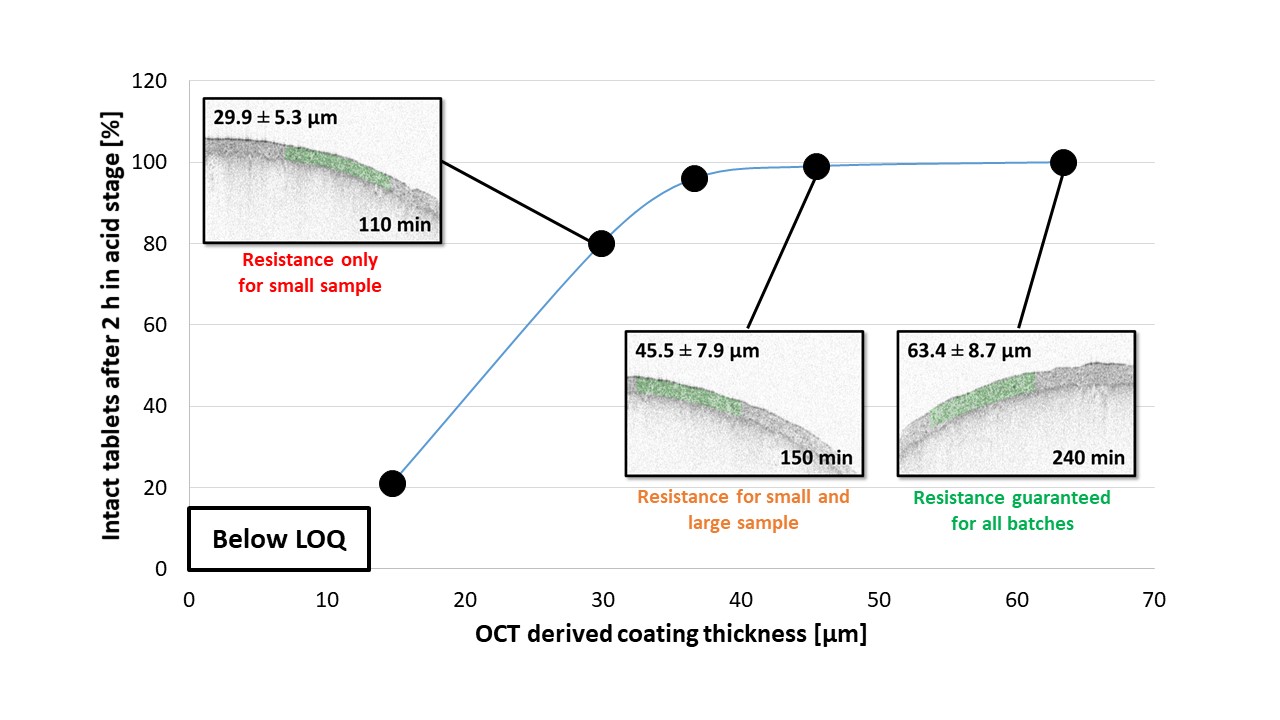
Figure 2: Percent of intact tablets as a function of actual coating thickness at coating time points 65 min, 110 min, 130 min, 150 min and 240 min (end) in batch A. Reported values are mean values ± standard deviation (including both aspects, convex surface and band of the tablets).
Manufacturing and Analytical Characterization - Chemical - Process Design and Controls
Category: Poster Abstract
(T1530-06-31) Determining the Minimum Required Thickness of Enteric Coatings by Means of Optical Coherence Tomography
Tuesday, October 18, 2022
3:30 PM – 4:30 PM ET
- SS
Sandra Stranzinger, Ph.D.
RCPE GmbH
Graz, Steiermark, Austria - SS
Sandra Stranzinger, Ph.D.
RCPE GmbH
Graz, Steiermark, Austria
Presenting Author(s)
Main Author(s)
Purpose: Enteric coatings are designed to protect active pharmaceutical ingredients (APIs) against untimely release in the stomach. It is commonly agreed that acid protection of such coatings depends on the coating layer thickness and integrity, which must be determined in an accurate and reliable way to ensure the desired final product’s performance. Optical coherence tomography (OCT) is an in-line capable technology that allows fast and non-destructive analysis of coating thickness and further material attributes via high-resolution cross-sectional images. A large set of data comprising the coating layer thickness and its uniformity, equally for single tablets (intra-sample coating variability) as well as between tablets (inter-sample coating variability), and the corresponding distributions, can be derived from OCT data. The merits of this technology to link derived coating material attributes to the respective acid protection function has been evaluated in the present study.
Methods: A classical pan coating process was used to apply an enteric coating based on a methacrylic copolymer aqueous dispersion. Three tablet batches (batch A, B and C) were investigated within this study. For each batch, five sample sets consisting of about 100 tablets each were drawn at selected coating time points during the run time of 240 min. An industrial OCT system equipped with a 1D-OCT probe (OSeeT Pharma 1D, Phyllon, Austria) combined with a spinning disc (Schuett-biotec, Germany) setup was used for off-line coating layer analyses. Multiple OCT images of the convex surfaces and the tablet bands of a large number of tablets (90 tablets) per coating time point were acquired for each batch to establish the coating thickness distribution of a representative number of tablets prior to testing their acid stage dissolution performance. In parallel, OCT measurements were performed on additional 6 tablets per coating time point for each batch before the USP dissolution testing of exact these tablets in order to facilitate direct comparison. OCT data and corresponding dissolution data of both samples sets (6 tablets and 90 tablets) were compared to assess their capability and relevance in order to predict a universal acid protection function of the investigated coating.
Results: The acquired data (OCT and dissolution) of the larger sample sets (90 tablets), enabled the calculation of a critical coating thickness required to protect the API from release in the stomach at given confidence interval. In particular, the coating time point exposing sufficient resistance to the gastric fluid was used (150 min, Figure 1), and derived functions used to calculate the critical coating thicknesses of the distribution that lie within a 3 sigma criterion. Accordingly, a critical coating thickness of 27.4 µm for a 3σ criterion could be determined. This information on the critical coating thickness would not have been accessible via the measured mean coating thickness and a standard deviation of 45.5 ± 7.9 µm taken from the corresponding small sample set (6 tablets, 150 min). The reason for this deviation is located in the inclined tailing of the distributions, showing a considerable number of thicknesses below the critical coating thickness for the distributions at earlier stages (i.e., 110 min, where only 80% of the tablets passed, Figure 2). Furthermore, in addition to existing functions used, a new distribution function could be proposed for improved description of the coating thickness distributions in the early stages of industrial coating processes.
Conclusion: It could be demonstrated that the use of OCT enables valuable insights into coating processes, which can be correlated with critical aspects of the drug performance (acid protection). The capabilities of OCT to provide substantial amounts of data on the applied coating thickness, including intra- and inter-sample coating variability and the coating thickness distribution, within seconds enable a more accurate acid protection function prediction. In a nutshell, the presented approach facilitates the calculation of a universal protection function for the investigated coating based on a sound statistical basis originating from the high amount of underlying OCT data. The proposed approach may be further transferred to in-line monitoring of the tablet coating processes, which could drastically improve the production efficiency by ultimately allowing real-time release testing (RTRT).
Acknowledgements: The Research Center Pharmaceutical Engineering (RCPE) is funded within the framework of COMET - Competence Centers for Excellent Technologies by BMK, BMDW, Land Steiermark and SFG. The COMET program is managed by the FFG.

Figure 1: Propagation of coating thickness distributions (mean values of convex surfaces and tablet bands) for the large samples (90 tablets, batch A). Different coating time points are indicated by different colors, the x-axis starts at 10 microns due to the limit of quantification of 12 µm for the OCT system used.

Figure 2: Percent of intact tablets as a function of actual coating thickness at coating time points 65 min, 110 min, 130 min, 150 min and 240 min (end) in batch A. Reported values are mean values ± standard deviation (including both aspects, convex surface and band of the tablets).
