Back
Purpose: This study aimed to develop a sublingual formulation of promethazine hydrochloride by direct compression, and to mask its intensely bitter taste.
Methods: Promethazine hydrochloride (PMZ) sublingual tablets prepared by direct compression were optimized using Box-Behnken full factorial design. The effect of a taste-masking agent (Eudragit E 100, X1), superdisintegrant (crospovidone; CPV, X2) and lubricant (sodium stearyl fumarate; SSF, X3) on sublingual tablets’ attributes (responses, Y) was optimized. The prepared sublingual tablets were characterized for hardness (Y1), disintegration time (Y2), initial dissolution rate (IDR; Y3) and dissolution efficiency after 30 min (Dissolution Efficiency (DE); Y4).
Results: The obtained results showed a significant positive effect of the three independent factors on tablet hardness (P < 0.05), and the interactive effect of Eudragit E 100 and CPV on tablet hardness was significant. Disintegration time was mainly affected by Eudragit E 100 and CPV concentrations. Moreover, IDR was employed to assess the taste masking effect, lower values were obtained at higher Eudragit E 100 concentration despite it was statistically insignificant (p > 0.05). Optimized formulation that was suggested by the software was composed of: Eudragit E 100 (X1) = 2.5% w/w, CPV (X2) = 4.13% w/w, and SSF (X3) = 1.0% w/w. The observed values of the optimized formula were found to be close to the predicted optimized values. The Differential Scanning Calorimetric (DSC) studies indicated no interaction between PMZ and tablet excipients.
Conclusion: The analysis of variance revealed that all the independent variables (individual, interactive or quadratic) had a significant effect on the hardness, disintegration time, and dissolution efficiency, but not the immediate dissolution rate. Novel PMZ-loaded sublingual tablets with very good properties were successfully produced. Optimal properties in terms of disintegration time ( < 20 s), hardness (around 2 kp), IDR of < 30% were obtained. This work has been able to produce a formulation of water-soluble drugs (as PMZ) in sublingual or orally dissolving tablets with optimal properties.
References: Suzuki A, Yasui-Furukori N, Mihara K, Kondo T, Furukori H, Inoue Y, Kaneko S, Otani K. Histamine H1-receptor antagonists, promethazine and homochlorcyclizine, increase the steady-state plasma concentrations of haloperidol and reduced haloperidol. Ther Drug Monit. 2003 Apr;25(2):192-6. doi: 10.1097/00007691-200304000-00008. PMID: 12657913.
Kolahian S, Jarolmasjed SH. Antiemetic efficacy of promethazine on xylazine-induced emesis in cats. Can Vet J. 2012;53(2):193-5.
Zur E. Nausea and vomiting in pregnancy: a review of the pathology and compounding opportunities. Int J Pharm Compd. 2013;17(2):113-23.
Fahler J, Wall GC, Leman BI. Gastroparesis-associated refractory nausea treated with aprepitant. Ann Pharmacother. 2012;46(12):e38.
Acknowledgements: The authors highly acknowledged the Ministry of Education and Deanship of Scientific Research, Najran University for funding this work ; Project Code [NU/MID/17/113]. Authors would like to acknowledge Kayyali chair for Pharmaceutical Industries, Department of Pharmaceutics, College of Pharmacy, King Saud University.
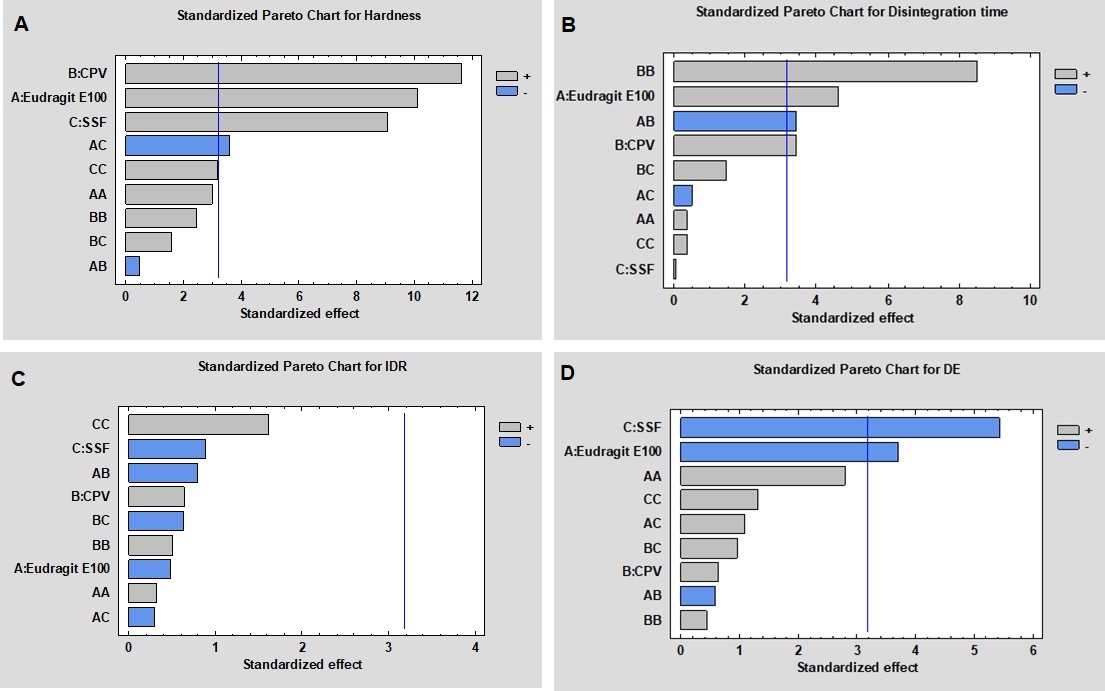
Standardized Pareto chart for the effect of independent variables on promethazine (PMZ) sublingual tablet hardness (A), disintegration time (B), Immediate dissolution rate (IDR) (C) and dissolution efficiency (DE) (D).
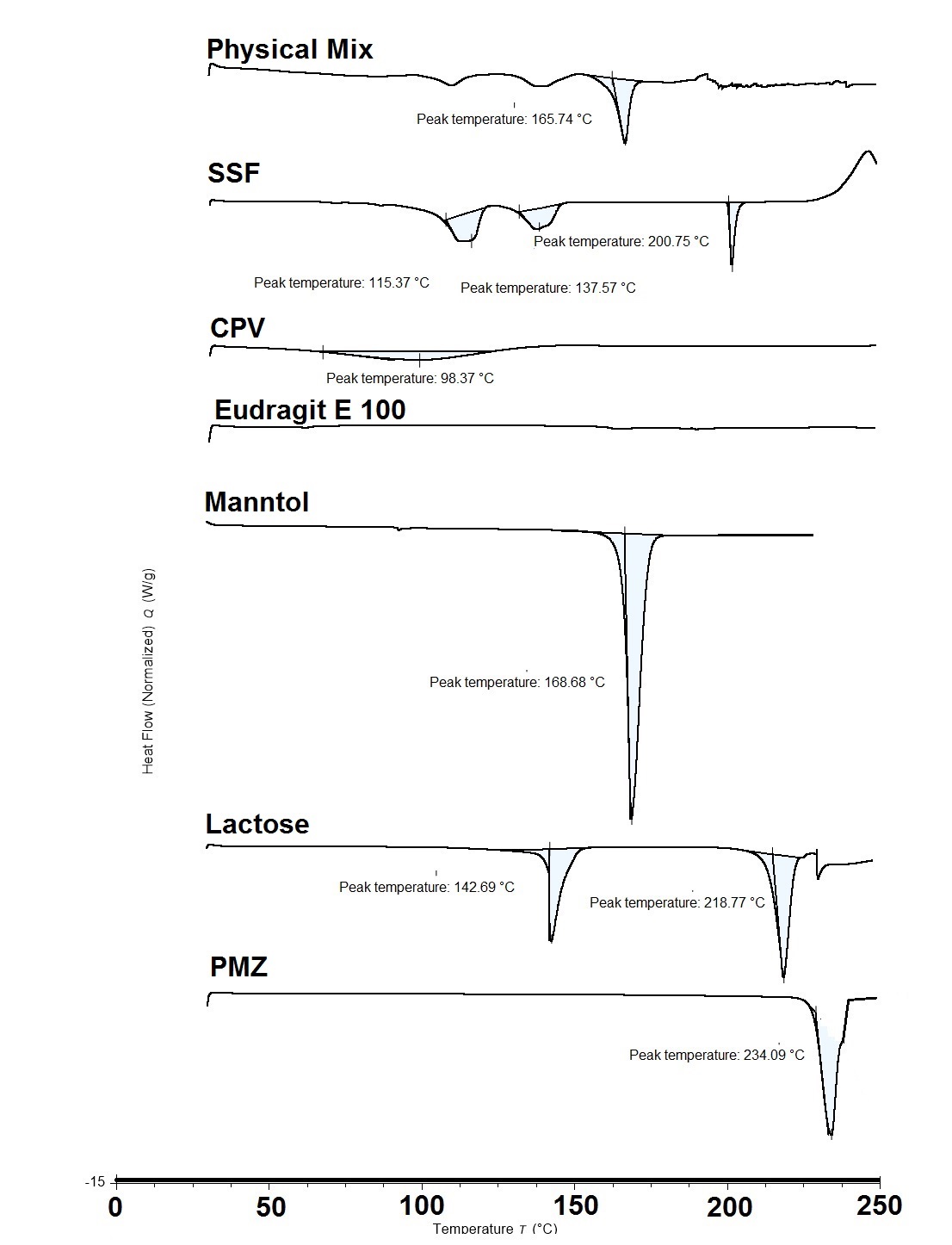
Differential scanning calorimetric scans of untreated promethazine (PMZ), lactose, mannitol, Eudragit E 100, crospovidone (CPV), sodium stearyl fumarate (SSF) and their physical mixture.
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(T1430-01-01) Development of Sublingual Tablets Containing Promethazine Hydrochloride for Paediatric Applications
Tuesday, October 18, 2022
2:30 PM – 3:30 PM ET

Hamad Alyami, PhD
Dr
Najran University
Najran, Najran, Saudi Arabia
Hamad Alyami, PhD
Dr
Najran University
Najran, Najran, Saudi Arabia
Presenting Author(s)
Main Author(s)
Purpose: This study aimed to develop a sublingual formulation of promethazine hydrochloride by direct compression, and to mask its intensely bitter taste.
Methods: Promethazine hydrochloride (PMZ) sublingual tablets prepared by direct compression were optimized using Box-Behnken full factorial design. The effect of a taste-masking agent (Eudragit E 100, X1), superdisintegrant (crospovidone; CPV, X2) and lubricant (sodium stearyl fumarate; SSF, X3) on sublingual tablets’ attributes (responses, Y) was optimized. The prepared sublingual tablets were characterized for hardness (Y1), disintegration time (Y2), initial dissolution rate (IDR; Y3) and dissolution efficiency after 30 min (Dissolution Efficiency (DE); Y4).
Results: The obtained results showed a significant positive effect of the three independent factors on tablet hardness (P < 0.05), and the interactive effect of Eudragit E 100 and CPV on tablet hardness was significant. Disintegration time was mainly affected by Eudragit E 100 and CPV concentrations. Moreover, IDR was employed to assess the taste masking effect, lower values were obtained at higher Eudragit E 100 concentration despite it was statistically insignificant (p > 0.05). Optimized formulation that was suggested by the software was composed of: Eudragit E 100 (X1) = 2.5% w/w, CPV (X2) = 4.13% w/w, and SSF (X3) = 1.0% w/w. The observed values of the optimized formula were found to be close to the predicted optimized values. The Differential Scanning Calorimetric (DSC) studies indicated no interaction between PMZ and tablet excipients.
Conclusion: The analysis of variance revealed that all the independent variables (individual, interactive or quadratic) had a significant effect on the hardness, disintegration time, and dissolution efficiency, but not the immediate dissolution rate. Novel PMZ-loaded sublingual tablets with very good properties were successfully produced. Optimal properties in terms of disintegration time ( < 20 s), hardness (around 2 kp), IDR of < 30% were obtained. This work has been able to produce a formulation of water-soluble drugs (as PMZ) in sublingual or orally dissolving tablets with optimal properties.
References: Suzuki A, Yasui-Furukori N, Mihara K, Kondo T, Furukori H, Inoue Y, Kaneko S, Otani K. Histamine H1-receptor antagonists, promethazine and homochlorcyclizine, increase the steady-state plasma concentrations of haloperidol and reduced haloperidol. Ther Drug Monit. 2003 Apr;25(2):192-6. doi: 10.1097/00007691-200304000-00008. PMID: 12657913.
Kolahian S, Jarolmasjed SH. Antiemetic efficacy of promethazine on xylazine-induced emesis in cats. Can Vet J. 2012;53(2):193-5.
Zur E. Nausea and vomiting in pregnancy: a review of the pathology and compounding opportunities. Int J Pharm Compd. 2013;17(2):113-23.
Fahler J, Wall GC, Leman BI. Gastroparesis-associated refractory nausea treated with aprepitant. Ann Pharmacother. 2012;46(12):e38.
Acknowledgements: The authors highly acknowledged the Ministry of Education and Deanship of Scientific Research, Najran University for funding this work ; Project Code [NU/MID/17/113]. Authors would like to acknowledge Kayyali chair for Pharmaceutical Industries, Department of Pharmaceutics, College of Pharmacy, King Saud University.

Standardized Pareto chart for the effect of independent variables on promethazine (PMZ) sublingual tablet hardness (A), disintegration time (B), Immediate dissolution rate (IDR) (C) and dissolution efficiency (DE) (D).

Differential scanning calorimetric scans of untreated promethazine (PMZ), lactose, mannitol, Eudragit E 100, crospovidone (CPV), sodium stearyl fumarate (SSF) and their physical mixture.
