Back
Purpose: Pulmonary delivery is a promising alternative for the delivery of protein medications such as antibodies to treat respiratory diseases. However, protein instability poses a major hurdle in formulation development. Consequently, pharmaceutical excipients, mainly sugars, are incorporated in protein formulations to maintain protein stability for extended periods of time. Mannitol is a widely used excipient incorporated in inhaled pharmaceuticals. The purpose of this study is to investigate the effect of varying concentrations of mannitol on the stability and aerodynamic performance of spray-dried protein formulations.
Methods: Bovine Serum Albumin (BSA) was used as a model protein and the formulations were characterized for their particle size using Malvern Mastersizer and Aerodynamic particle size using Next Generation Impactor (NGI). Additionally, the particles were also characterized with solid-state Fourier-transform infrared spectroscopy, powder X-ray diffraction, scanning electron microscopy, and BET to analyze the change in their secondary structure, crystallinity, particle morphology respectively, and surface area. The physical stability of formulations was studied using Size exclusion chromatography (SEC).
Results: The particle size of all the spray-dried protein formulations was found to be between 1 – 5 µm , indicating that protein formulations were inhalable. The moisture content of all protein formulations was about 5 % w/w. Formulations with higher (greater than 30% by weight) concentrations of mannitol showed crystallization tendencies on storage for 60 days (Figure 1). This resulted in increased monomer loss with time as demonstrated by the stability study. BSA: Mannitol – 5:1 was found to be relatively more stable (lower monomer loss) than all other BSA: Mannitol formulations (Figure 2). Fine Particle Fraction (FPF) as determined using NGI also decreased with time for formulations with relatively high mannitol concentrations (Figure 3). FPF for 100% BSA and BSA: Mannitol – 5:1, remained unchanged (FPF = 67 ± 3.57) for 60 days, suggesting no agglomeration tendencies for these formulations (Figure 3). Interestingly, data obtained from SEC and NGI suggested that protein aggregation did not cause particle agglomeration, as evident by the monomer loss for 100% BSA formulation in the stability study, but unchanged FPF for 100% BSA formulation in NGI (Figure 2 and Figure 3). The decrease in FPF was most likely due to crystallization tendencies of mannitol. BSA: Mannitol – 5:1 remained amorphous for an extended period (60 days), this could be due to higher BSA concentration which inhibits crystallization tendencies of mannitol.
Conclusion: Protein formulations made varying mannitol ratios were inhalable. However, mannitol when used in high concentrations does not stabilize protein due to its crystallization tendencies. This study improves our understanding of the concentration-dependent effect of mannitol on aerosol performance and stability of inhaled protein formulations
References: 1. Chen, Y., Mutukuri, T. T., Wilson, N. E., & Zhou, Q. (Tony). (2021). Pharmaceutical protein solids: Drying technology, solid-state characterization and stability. Advanced Drug Delivery Reviews, 172, 211–233.
2. K, I., S, Y., & T, T. (1994). Effect of mannitol crystallinity on the stabilization of enzymes during freeze-drying. Chemical & Pharmaceutical Bulletin, 42(1), 5–8.
3. Zhou, Q. T., Tang, P., Leung, S. S. Y., Chan, J. G. Y., & Chan, H. K. (2014). Emerging inhalation aerosol devices and strategies: Where are we headed? Advanced Drug Delivery Reviews, 75, 3–17.
Acknowledgments: Purdue University, Department of Industrial and Physical Pharmacy, Center for Pharmaceutical Processing Research (CPPR), Center for Pharmaceutical Development (CPD)
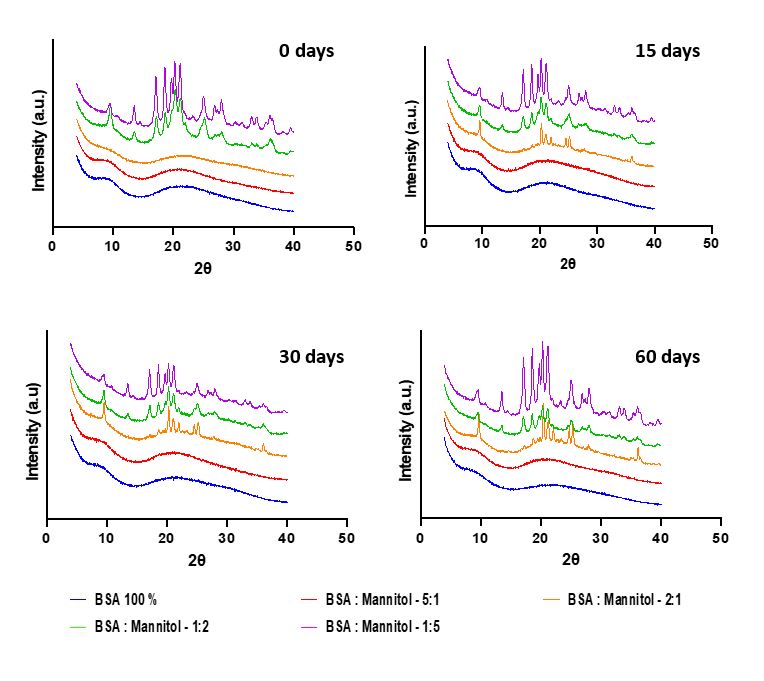
Figure 1. X-Ray Diffraction (XRD) plot for protein formulations carried out using Empyrean X-ray diffractometer (Malvern Panalytical, Royston, United Kingdom). Samples were analyzed in the 2θ angle range of 5° to 50 °.
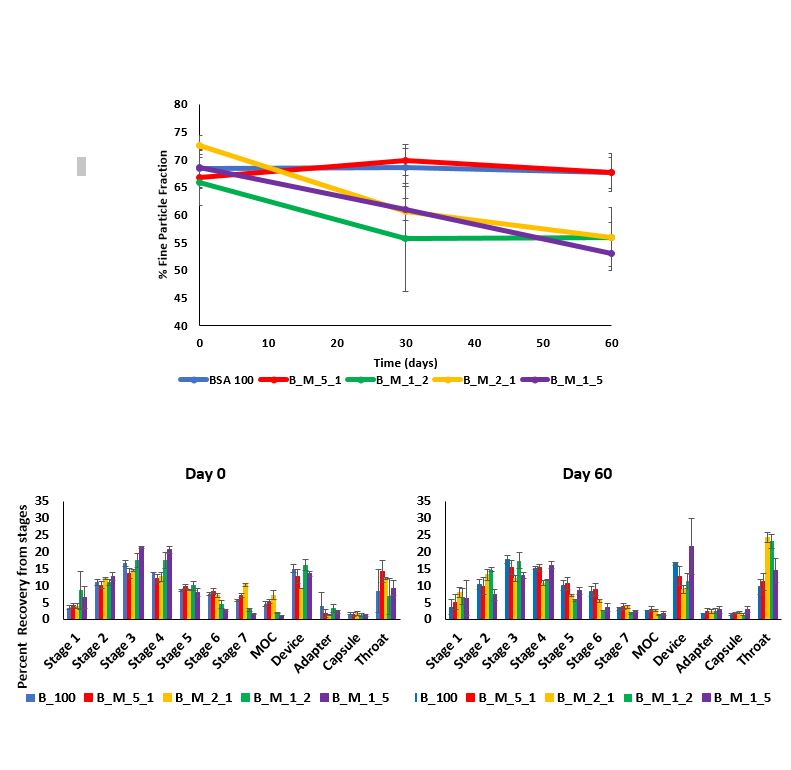
Figure 3. Changes in the fine particle fractions (FPF) of BSA Mannitol formulations representing the mass of drug deposited on the impactor stages with the cut-off diameter less than or equal to 5µm over 60 days
Figure 2. Physical stability of BSA Mannitol formulations on storage at 40 °C for 60 days (n = 3, mean ± SD
Formulation and Delivery - Biomolecular - Formulation
Category: Late Breaking Poster Abstract
(W1230-07-37) Understanding the Effects of Varying Mannitol Concentrations on Inhalable Protein Formulations
Wednesday, October 19, 2022
12:30 PM – 1:30 PM ET
- KA
Kinnari Arte, MS
Purdue University
West Lafayette, Indiana, United States - KA
Kinnari Arte, MS
Purdue University
West Lafayette, Indiana, United States
Presenting Author(s)
Main Author(s)
Purpose: Pulmonary delivery is a promising alternative for the delivery of protein medications such as antibodies to treat respiratory diseases. However, protein instability poses a major hurdle in formulation development. Consequently, pharmaceutical excipients, mainly sugars, are incorporated in protein formulations to maintain protein stability for extended periods of time. Mannitol is a widely used excipient incorporated in inhaled pharmaceuticals. The purpose of this study is to investigate the effect of varying concentrations of mannitol on the stability and aerodynamic performance of spray-dried protein formulations.
Methods: Bovine Serum Albumin (BSA) was used as a model protein and the formulations were characterized for their particle size using Malvern Mastersizer and Aerodynamic particle size using Next Generation Impactor (NGI). Additionally, the particles were also characterized with solid-state Fourier-transform infrared spectroscopy, powder X-ray diffraction, scanning electron microscopy, and BET to analyze the change in their secondary structure, crystallinity, particle morphology respectively, and surface area. The physical stability of formulations was studied using Size exclusion chromatography (SEC).
Results: The particle size of all the spray-dried protein formulations was found to be between 1 – 5 µm , indicating that protein formulations were inhalable. The moisture content of all protein formulations was about 5 % w/w. Formulations with higher (greater than 30% by weight) concentrations of mannitol showed crystallization tendencies on storage for 60 days (Figure 1). This resulted in increased monomer loss with time as demonstrated by the stability study. BSA: Mannitol – 5:1 was found to be relatively more stable (lower monomer loss) than all other BSA: Mannitol formulations (Figure 2). Fine Particle Fraction (FPF) as determined using NGI also decreased with time for formulations with relatively high mannitol concentrations (Figure 3). FPF for 100% BSA and BSA: Mannitol – 5:1, remained unchanged (FPF = 67 ± 3.57) for 60 days, suggesting no agglomeration tendencies for these formulations (Figure 3). Interestingly, data obtained from SEC and NGI suggested that protein aggregation did not cause particle agglomeration, as evident by the monomer loss for 100% BSA formulation in the stability study, but unchanged FPF for 100% BSA formulation in NGI (Figure 2 and Figure 3). The decrease in FPF was most likely due to crystallization tendencies of mannitol. BSA: Mannitol – 5:1 remained amorphous for an extended period (60 days), this could be due to higher BSA concentration which inhibits crystallization tendencies of mannitol.
Conclusion: Protein formulations made varying mannitol ratios were inhalable. However, mannitol when used in high concentrations does not stabilize protein due to its crystallization tendencies. This study improves our understanding of the concentration-dependent effect of mannitol on aerosol performance and stability of inhaled protein formulations
References: 1. Chen, Y., Mutukuri, T. T., Wilson, N. E., & Zhou, Q. (Tony). (2021). Pharmaceutical protein solids: Drying technology, solid-state characterization and stability. Advanced Drug Delivery Reviews, 172, 211–233.
2. K, I., S, Y., & T, T. (1994). Effect of mannitol crystallinity on the stabilization of enzymes during freeze-drying. Chemical & Pharmaceutical Bulletin, 42(1), 5–8.
3. Zhou, Q. T., Tang, P., Leung, S. S. Y., Chan, J. G. Y., & Chan, H. K. (2014). Emerging inhalation aerosol devices and strategies: Where are we headed? Advanced Drug Delivery Reviews, 75, 3–17.
Acknowledgments: Purdue University, Department of Industrial and Physical Pharmacy, Center for Pharmaceutical Processing Research (CPPR), Center for Pharmaceutical Development (CPD)

Figure 1. X-Ray Diffraction (XRD) plot for protein formulations carried out using Empyrean X-ray diffractometer (Malvern Panalytical, Royston, United Kingdom). Samples were analyzed in the 2θ angle range of 5° to 50 °.

Figure 3. Changes in the fine particle fractions (FPF) of BSA Mannitol formulations representing the mass of drug deposited on the impactor stages with the cut-off diameter less than or equal to 5µm over 60 days

Figure 2. Physical stability of BSA Mannitol formulations on storage at 40 °C for 60 days (n = 3, mean ± SD
