Back
Purpose: Method migration or moving methods from one system to another can be challenging due to differences in design and method settings across systems. Learning about the similarities and differences between system designs, and how these can impact method migration, will allow users to move methods between systems with a higher level of confidence. For example, the differences in autosampler characteristics can impact carryover, leading to failure to meet system suitability requirements or to inaccurate quantitative results. To reduce variability across systems, understanding and optimizing needle wash settings is essential in method migration. This investigation will focus on needle wash differences between two UHPLC systems. Both systems utilize a flow-thru-needle autosampler, while one system includes a dual needle configuration. Comparison will be performed between the systems which have either pre and post-injection wash options or a needle wash design that only allows for pre-injection and post-aspirate washing. Additional autosampler characteristics will also be evaluated, including cycle time and its relationship to settings to reduce carryover.
Methods: Carryover can be challenging, particularly for those compounds that may adsorb or adhere to different surfaces of an LC autosampler. In this study, multiple carryover methods were used to compare the carryover performance of two UHPLC systems. One method evaluated carryover of a small molecule analyte (caffeine) that is less likely to adhere to the LC surface, but is often used to evaluate volumetric carryover. A second method evaluated carryover of chlorohexidine, which is more likely to interact or absorb to the surface of an LC. Both methodologies included injecting a challenge sample (at a high concentration) followed by blanks to assess carryover. Quantification, when possible, was performed using standards at the linear range from the limit of quantitation (LOQ) up to approximately 50x concentration. All tests were repeated in triplicate and various settings were evaluated to determine the impact on carryover. In addition, the impact of the various settings on the injection cycle times was also evaluated. In addition to the performance testing, a method based on the USP Monograph for chlorhexidine organic impurities was also analyzed on both systems to evaluate the impact of carryover performance during method migration of a UHPLC method. The USP method conditions include a gradient with 0.1% trifluoracetic acid (TFA) in water and 0.1% TFA in acetonitrile as well as a sample concentration at 11.4mg/ml.
Results: As described above both volumetric and adsorptive carryover were evaluated. For volumetric, carryover was well below system specifications. The needle wash was not found to have a significant impact; however, adjusting washing parameters was found to impact both volumetric carryover and cycle time. For those conditions in which carryover was observed, subsequent injections diluted and/or eliminated carryover. In contrast, absorptive carryover was found to be impacted by needle wash composition as well as the specific wash settings. As expected, stronger carryover washes resulted in a significant reduction in carryover.
Conclusion: Method transfer between different UHPLC systems can be challenging due to differences in design. It is important to consider the differences between systems including autosampler design, and its impact on method performance including carryover. In this presentation, the impact of design differences and method settings on the autosampler were assessed to determine the impact on carryover performance and cycle time. The study demonstrated those adjustments that can be made to ensure similar performance across systems and reduce differences in carryover across systems. This study also demonstrated how to maximize wash efficiency without impacting overall cycle time.
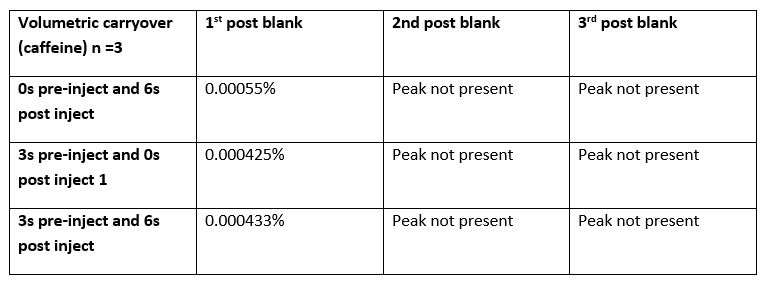
Table 1: Volumetric carryover measurements (Caffeine), Conditions: Isocratic gradient 90:10 water: acetonitrile. Challenge concentration 4mg/ml caffeine.
Bioanalytics - Biomolecular - Drug Quantification
Category: Late Breaking Poster Abstract
(T1530-09-52) Impact of Autosampler Design on Carryover Performance During Method Migration
Tuesday, October 18, 2022
3:30 PM – 4:30 PM ET
- PH
Paula Hong, Ph.D.
Waters Corporation
Milford, Massachusetts, United States - NK
Nicole Kramer, BS
Waters Corporation
Milford, Massachusetts, United States
Presenting Author(s)
Main Author(s)
Purpose: Method migration or moving methods from one system to another can be challenging due to differences in design and method settings across systems. Learning about the similarities and differences between system designs, and how these can impact method migration, will allow users to move methods between systems with a higher level of confidence. For example, the differences in autosampler characteristics can impact carryover, leading to failure to meet system suitability requirements or to inaccurate quantitative results. To reduce variability across systems, understanding and optimizing needle wash settings is essential in method migration. This investigation will focus on needle wash differences between two UHPLC systems. Both systems utilize a flow-thru-needle autosampler, while one system includes a dual needle configuration. Comparison will be performed between the systems which have either pre and post-injection wash options or a needle wash design that only allows for pre-injection and post-aspirate washing. Additional autosampler characteristics will also be evaluated, including cycle time and its relationship to settings to reduce carryover.
Methods: Carryover can be challenging, particularly for those compounds that may adsorb or adhere to different surfaces of an LC autosampler. In this study, multiple carryover methods were used to compare the carryover performance of two UHPLC systems. One method evaluated carryover of a small molecule analyte (caffeine) that is less likely to adhere to the LC surface, but is often used to evaluate volumetric carryover. A second method evaluated carryover of chlorohexidine, which is more likely to interact or absorb to the surface of an LC. Both methodologies included injecting a challenge sample (at a high concentration) followed by blanks to assess carryover. Quantification, when possible, was performed using standards at the linear range from the limit of quantitation (LOQ) up to approximately 50x concentration. All tests were repeated in triplicate and various settings were evaluated to determine the impact on carryover. In addition, the impact of the various settings on the injection cycle times was also evaluated. In addition to the performance testing, a method based on the USP Monograph for chlorhexidine organic impurities was also analyzed on both systems to evaluate the impact of carryover performance during method migration of a UHPLC method. The USP method conditions include a gradient with 0.1% trifluoracetic acid (TFA) in water and 0.1% TFA in acetonitrile as well as a sample concentration at 11.4mg/ml.
Results: As described above both volumetric and adsorptive carryover were evaluated. For volumetric, carryover was well below system specifications. The needle wash was not found to have a significant impact; however, adjusting washing parameters was found to impact both volumetric carryover and cycle time. For those conditions in which carryover was observed, subsequent injections diluted and/or eliminated carryover. In contrast, absorptive carryover was found to be impacted by needle wash composition as well as the specific wash settings. As expected, stronger carryover washes resulted in a significant reduction in carryover.
Conclusion: Method transfer between different UHPLC systems can be challenging due to differences in design. It is important to consider the differences between systems including autosampler design, and its impact on method performance including carryover. In this presentation, the impact of design differences and method settings on the autosampler were assessed to determine the impact on carryover performance and cycle time. The study demonstrated those adjustments that can be made to ensure similar performance across systems and reduce differences in carryover across systems. This study also demonstrated how to maximize wash efficiency without impacting overall cycle time.

Table 1: Volumetric carryover measurements (Caffeine), Conditions: Isocratic gradient 90:10 water: acetonitrile. Challenge concentration 4mg/ml caffeine.
