Back
Purpose: Inhalation therapy is an effective and safe means to treat lung diseases by delivering drugs directly to the airways. Certain lung and chronic airways diseases (e.g., chronic sinusitis, asthma, COPD, cystic fibrosis, and bronchiectasis) could be treated with high molecular weight (HMW) hyaluronic acid ( >1,000 kDa), due to its anti-inflammatory and water-binding capacities. Indeed, the literature reports several clinical studies in which the inhalation of hyaluronic acid (HA) solutions leads to positive clinical outcomes. However, in those studies there is little if any attention to the aerodynamic performance of nebulized solution of HA. High viscosity severely limits the nebulization and therefore dose delivered into deep lung. In order to fill this gap, we investigated in vitro how HMW hyaluronan (NaHA) solutions, with and without surfactants, perform from an aerodynamic perspective, interplay of viscosity and surface tension to facilitate nebulization. Considering the scarcity of excipients for pulmonary application, non-ionic surfactant with least immunogenicity were investigated. There are no report of such investigation of highly viscous anti-inflammatory polymeric solution in pulmonary delivery.
Methods: The in vitro deposition profile and aerosol properties (according to Ph.Eur. 2.9.18) of NaHA (1,330 kDa) solutions in water (Table I), with or without surfactants. Methylene blue dye was used as a marker for quantification purposes (n=3). The M170 Next Generation ImpactorTM (NGI) connected to PARI LC Plus® nebulizer cup and PARI PRONEB Ultra II compressor system was used. The nebulizer was activated for 4 min at a flow rate of 15 L/min and the amount of dye deposited on each NGI stage was collected with water and quantified by UV spectrometry. The amount of dye deposited in the impactor allowed for the calculation of the following aerosol parameters: emitted fraction (EF), fine particle fraction (FPF), Median Aerodynamic Diameter (MMAD) and the Geometric Standard Deviation (GSD). The viscosity was measured by rotational viscometer equipped with spindles. Biocompatibility of HA+surfactant system was evaluated using A549 (human lung carcinoma cells) and RAW 264.7 (macrophage) using MTT assay. Currently, we are investigating cytokine production on exposure to HA+surfactant combination in macrophage.
Results: The therapeutic potential of inhaled HMW hyaluronan is well established, but its molecular weight leads to highly viscous solutions hindering nebulization. The more viscous the fluid, the longer the nebulization time, associated with increased residual amounts in the cup (lower outputs). Nebulization of the non-viscous solution of water and dye (#1 in Table I), gave an emitted fraction (EF) of 15.3±1.2%, which could be increased to 18.7% with addition of 0.02% Tween 80 (solution #2, n=1). Furthermore, the output also improved from about 25% to 34%, respectively for solution #1 and #6. Conversely, not unexpectedly, the nebulization of 1.5 mg/ml NaHA solution without surfactant (#3 in Table) drastically decreased the output to 13% with most solution remaining in the nebulizer’s cup, likely due its viscosity (Table II). The EF was 2.4±0.7%. Together with EF, the main quality attribute affecting the clinical efficacy of an inhaler, is the fine particle mass (i.e., the actual active dose), corresponding to the fraction of the emitted dose whose aerodynamic size is lower than 5 μm (FPF). These fine particles are able to penetrate and deposit deep into the lung. For the 1.5 mg/ml NaHA solution, FPF was very good around 82%. In order to improve the output while keeping NaHA concentration constant, increasing concentrations of Tween 80 were added. With both surfactant concentrations (0.02% and 0.2%) a better output was achieved as the surfactant addition lowered the solution’s viscosity. The emitted fraction increased with the increase of surfactant concentration in the solution, despite 0.2% Tween 80 (solution #5 in Table I) did not further decreased the viscosity compared to 0.02% Tween 80 (solution #4 in Table I). In fact, the viscosity of solutions #4-5 was almost the same (Table II). Altogether, the results show that addition of surfactants could be a strategy to achieve a better nebulization of viscous NaHA solutions. Noteworthy, the widely used Tween 80 surfactant poses immunogenicity issues. In this regard, 0.2% Kolliphor HS 15 was considered as an alternative surfactant (solution #6 in Table I), achieving slightly better output in comparison with 0.2% Tween 80, and similar EF and FPF (Table II). All formulations were effective in producing respirable aerosols, whose aerodynamic size distributions are shown in Figure 1. MMAD values were < 5 μm in all cases. HA with 0.2% w/v Kolliphor HS 15 was found to be compatible with A549 and RAW cells.
Conclusion: Overall, this study shows how to improve nebulization HA solutions for inhalation in chronic lung diseases. High viscosity of HMW NaHA drastically hampers nebulization efficiency but addition of very low concentrations of non-immunogenic surfactant - Kolliphor HS 15 could significantly increase emitted fraction and achieve clinically meaningful dose in the deep lung.
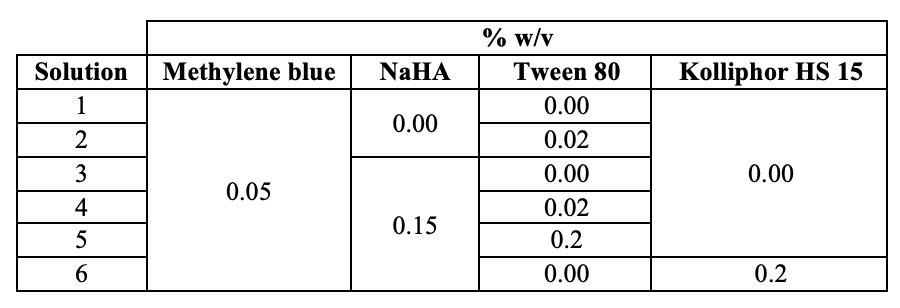
Table I. Hyaluronan (NaHA) solutions composition by weight/volume.
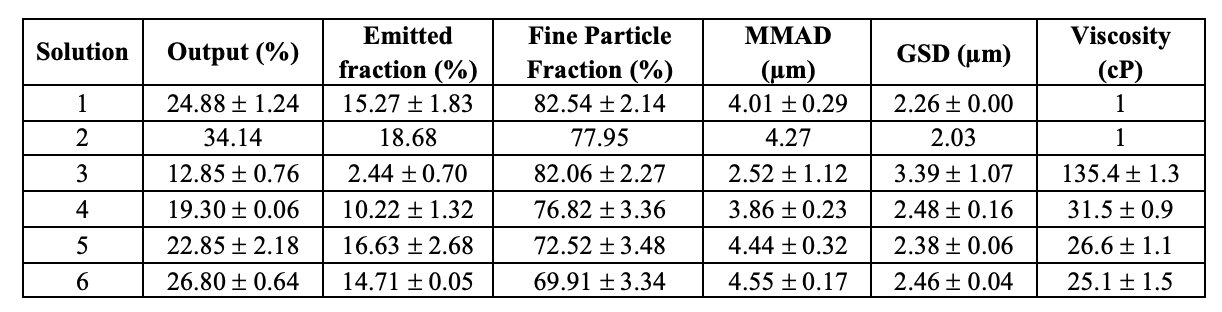
Table II. Aerosol properties of hyaluronan formulations aerosolized inside the Next Generation Impactor at 15 L/min and viscosity measured by rotational viscometer. Data represent mean ± SD (n = 3).
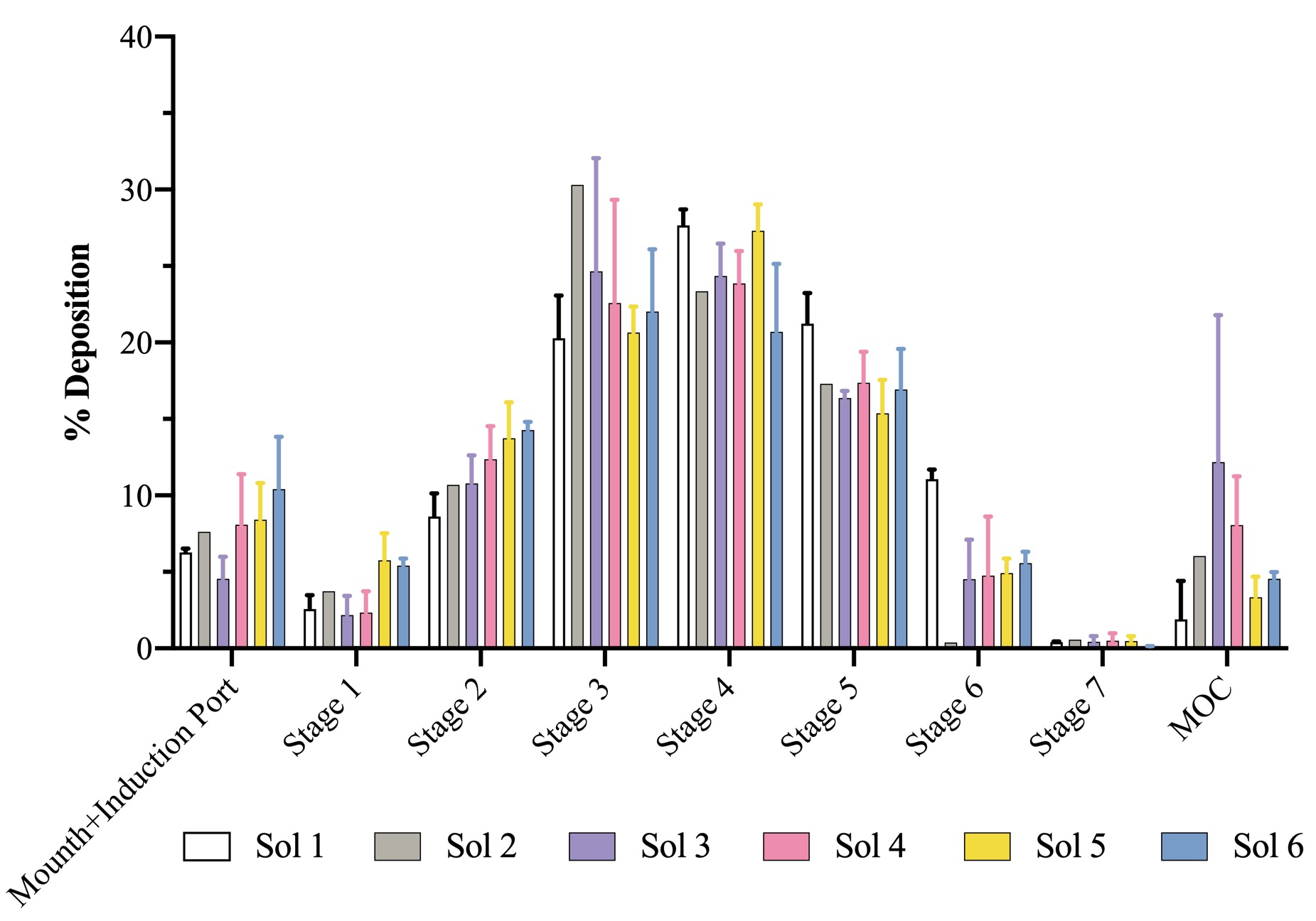
Figure 1. Aerodynamic size distribution of all solutions in Next-Gen Impactor™ (NGI). Data represent mean ± SD (n = 3).
Formulation and Delivery - Biomolecular - Drug Delivery
Category: Late Breaking Poster Abstract
(M1530-03-13) A Strategy to Deliver High Molecular Weight Hyaluronic Acid in Deep Lung: Investigating the Role of Non-immunogenic Surfactant
Monday, October 17, 2022
3:30 PM – 4:30 PM ET
- SB
Sabrina Banella
University of Ferrara
Ferrara, Emilia-Romagna, Italy - SB
Sabrina Banella
University of Ferrara
Ferrara, Emilia-Romagna, Italy
Presenting Author(s)
Main Author(s)
Purpose: Inhalation therapy is an effective and safe means to treat lung diseases by delivering drugs directly to the airways. Certain lung and chronic airways diseases (e.g., chronic sinusitis, asthma, COPD, cystic fibrosis, and bronchiectasis) could be treated with high molecular weight (HMW) hyaluronic acid ( >1,000 kDa), due to its anti-inflammatory and water-binding capacities. Indeed, the literature reports several clinical studies in which the inhalation of hyaluronic acid (HA) solutions leads to positive clinical outcomes. However, in those studies there is little if any attention to the aerodynamic performance of nebulized solution of HA. High viscosity severely limits the nebulization and therefore dose delivered into deep lung. In order to fill this gap, we investigated in vitro how HMW hyaluronan (NaHA) solutions, with and without surfactants, perform from an aerodynamic perspective, interplay of viscosity and surface tension to facilitate nebulization. Considering the scarcity of excipients for pulmonary application, non-ionic surfactant with least immunogenicity were investigated. There are no report of such investigation of highly viscous anti-inflammatory polymeric solution in pulmonary delivery.
Methods: The in vitro deposition profile and aerosol properties (according to Ph.Eur. 2.9.18) of NaHA (1,330 kDa) solutions in water (Table I), with or without surfactants. Methylene blue dye was used as a marker for quantification purposes (n=3). The M170 Next Generation ImpactorTM (NGI) connected to PARI LC Plus® nebulizer cup and PARI PRONEB Ultra II compressor system was used. The nebulizer was activated for 4 min at a flow rate of 15 L/min and the amount of dye deposited on each NGI stage was collected with water and quantified by UV spectrometry. The amount of dye deposited in the impactor allowed for the calculation of the following aerosol parameters: emitted fraction (EF), fine particle fraction (FPF), Median Aerodynamic Diameter (MMAD) and the Geometric Standard Deviation (GSD). The viscosity was measured by rotational viscometer equipped with spindles. Biocompatibility of HA+surfactant system was evaluated using A549 (human lung carcinoma cells) and RAW 264.7 (macrophage) using MTT assay. Currently, we are investigating cytokine production on exposure to HA+surfactant combination in macrophage.
Results: The therapeutic potential of inhaled HMW hyaluronan is well established, but its molecular weight leads to highly viscous solutions hindering nebulization. The more viscous the fluid, the longer the nebulization time, associated with increased residual amounts in the cup (lower outputs). Nebulization of the non-viscous solution of water and dye (#1 in Table I), gave an emitted fraction (EF) of 15.3±1.2%, which could be increased to 18.7% with addition of 0.02% Tween 80 (solution #2, n=1). Furthermore, the output also improved from about 25% to 34%, respectively for solution #1 and #6. Conversely, not unexpectedly, the nebulization of 1.5 mg/ml NaHA solution without surfactant (#3 in Table) drastically decreased the output to 13% with most solution remaining in the nebulizer’s cup, likely due its viscosity (Table II). The EF was 2.4±0.7%. Together with EF, the main quality attribute affecting the clinical efficacy of an inhaler, is the fine particle mass (i.e., the actual active dose), corresponding to the fraction of the emitted dose whose aerodynamic size is lower than 5 μm (FPF). These fine particles are able to penetrate and deposit deep into the lung. For the 1.5 mg/ml NaHA solution, FPF was very good around 82%. In order to improve the output while keeping NaHA concentration constant, increasing concentrations of Tween 80 were added. With both surfactant concentrations (0.02% and 0.2%) a better output was achieved as the surfactant addition lowered the solution’s viscosity. The emitted fraction increased with the increase of surfactant concentration in the solution, despite 0.2% Tween 80 (solution #5 in Table I) did not further decreased the viscosity compared to 0.02% Tween 80 (solution #4 in Table I). In fact, the viscosity of solutions #4-5 was almost the same (Table II). Altogether, the results show that addition of surfactants could be a strategy to achieve a better nebulization of viscous NaHA solutions. Noteworthy, the widely used Tween 80 surfactant poses immunogenicity issues. In this regard, 0.2% Kolliphor HS 15 was considered as an alternative surfactant (solution #6 in Table I), achieving slightly better output in comparison with 0.2% Tween 80, and similar EF and FPF (Table II). All formulations were effective in producing respirable aerosols, whose aerodynamic size distributions are shown in Figure 1. MMAD values were < 5 μm in all cases. HA with 0.2% w/v Kolliphor HS 15 was found to be compatible with A549 and RAW cells.
Conclusion: Overall, this study shows how to improve nebulization HA solutions for inhalation in chronic lung diseases. High viscosity of HMW NaHA drastically hampers nebulization efficiency but addition of very low concentrations of non-immunogenic surfactant - Kolliphor HS 15 could significantly increase emitted fraction and achieve clinically meaningful dose in the deep lung.

Table I. Hyaluronan (NaHA) solutions composition by weight/volume.

Table II. Aerosol properties of hyaluronan formulations aerosolized inside the Next Generation Impactor at 15 L/min and viscosity measured by rotational viscometer. Data represent mean ± SD (n = 3).

Figure 1. Aerodynamic size distribution of all solutions in Next-Gen Impactor™ (NGI). Data represent mean ± SD (n = 3).
