Back
Purpose: Long-acting implantables (LAIs) have become increasingly important in providing novel formulations for active pharmaceutical ingredients (APIs). Traditionally, these systems have utilized polymers such as poly(lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), and ethylene vinyl acetate (EVA). To provide sustained release, these polymers often rely on diffusion-based mechanisms where PLGA and PCL exhibit bulk-eroding degradation that results in a high burst release and less predictable mass loss and drug delivery. Biodegradable poly(glycerol sebacate) urethane--HydraleseTM (PGSU)—is a novel elastomer that may be used for the next generation of LAIs. PGSU is unique in its ability to provide linear release kinetics, high drug loading (often >60% w/w), and a surface-eroding degradation mechanism that minimizes burst release. Furthermore, PGSU maintains excellent mechanical properties such as high flexibility, shelf stability under ambient conditions, and biocompatibility. Ocular therapeutics often find benefit from controlled release dosage forms. Patient compliance is often low with the frequent dosing regimen that is prescribed with many ophthalmic medications (often several times per day for eyedrops, or weekly or monthly injections, depending on ocular disease and indication). Patient tolerability and safety are especially important considerations for repeat dosing. The immune-privileged nature of the eye allows for the direct implantation of a variety of controlled release dosage forms without some of the challenges faced in other areas of the body. From a manufacturing perspective, these intra-ocular devices must be made small enough to be delivered using appropriate gauge needles (often requiring diameters of 450um or less) and must be mechanically robust enough to be handled during drug loading and manufacturing. From a therapeutic perspective, intra-ocular devices must provide sufficient duration of treatment, must not negatively impact vision or physiological function, and must be eventually degradable. The controlled release, non-swelling, flexible, and biocompatible benefits of PGSU, led our group to investigate the development and characterization of PGSU microdevices intended for intra-vitreal administration.
Methods: PGS resin was utilized to manufacture drug-loaded Hydralese (PGSU) microdevices approximately 300 μm in diameter and 10 mm in length. In this study, we investigated a 10% w/w final loading of dexamethasone using multiple different pre-polymer formulations. Dexamethasone was selected as an API for these systems due to its utility in the treatment of various ocular diseases, such as diabetic macular edema and uveitis. A two-component dual-barrel cartridge system was utilized to manufacture microdevices. Process mixing uniformity and final product content uniformity were evaluated using solvent extraction procedures followed by quantification with high performance liquid chromatography (HPLC). To identify spatial content uniformity of samples, a Raman microscope equipped with a 785nm laser was utilized. Microdevices were spatially mapped by gathering spectra at various points along each device length, then analyzed to determine area under the curve for Raman bands associated with dexamethasone. Drug release studies were conducted on n=3 microdevices formulated using 10% w/w dexamethasone loading. Microdevices were incubated in 1mL of 0.1M PBS at 37 °C under gentle agitation. Media was sampled and replaced weekly, and releaseates were quantified for dexamethasone concentration using HPLC. Final microdevice constructs were analyzed post-dissolution using extraction methods described previously and SEM to assess drug release and material degradation.
Results: Microdevices manufactured using this technique could be fabricated at a variety of sizes ranging from as small as 200 μm in diameter (Figure 1) and up to 70% w/w API loading. Three formulations of 10% w/w dexamethasone loading were manufactured at 300 μm diameter. These formulations varied the distribution of API in the A and B component mixes to determine which would result in optimal mixing. In-process samples were taken of A component and B component polymer mixes and assessed for content uniformity. Across 5 samples in each mix, drug loading was assessed to be >98% of theoretical loading with relative standard deviations of < 1.5%, indicating complete and uniform mixing (Figure 2A). Content uniformity of final microdevices assessed via Raman microscopy also showed consistent spatial distribution of API throughout the length of these microdevices (Figure 2B). Finally, extraction of microdevices sampled throughout the manufacturing batch indicated uniform API content at all points during manufacturing. Microdevices of each formulation (n=3) were assayed for in vitro release over 10 weeks. Comparison of pre- and post-release cross-sectional SEM images showed dissolution and release of API particles. Analysis of microdevice surface topographies showed slight pitting after 10 weeks of in vitro release, which indicates early signs of polymer erosion. Notably, no polymer swelling was observed, which is a benefit of the PGSU crosslinked polymer network. Finally, these constructs provided sustained release of dexamethasone for around 8 weeks prior to depletion of payload.
Conclusion: PGSU is a useful polymer excipient in the manufacture of drug-loaded microdevices for use as intra-vitreal implants. Manufacturing processes for PGSU materials support the fabrication of uniform, flexible, and small diameter devices that can sustain the release of dexamethasone for at least 8 weeks in vitro.

Drug loaded PGSU microdevices formed using a variety of diameters.
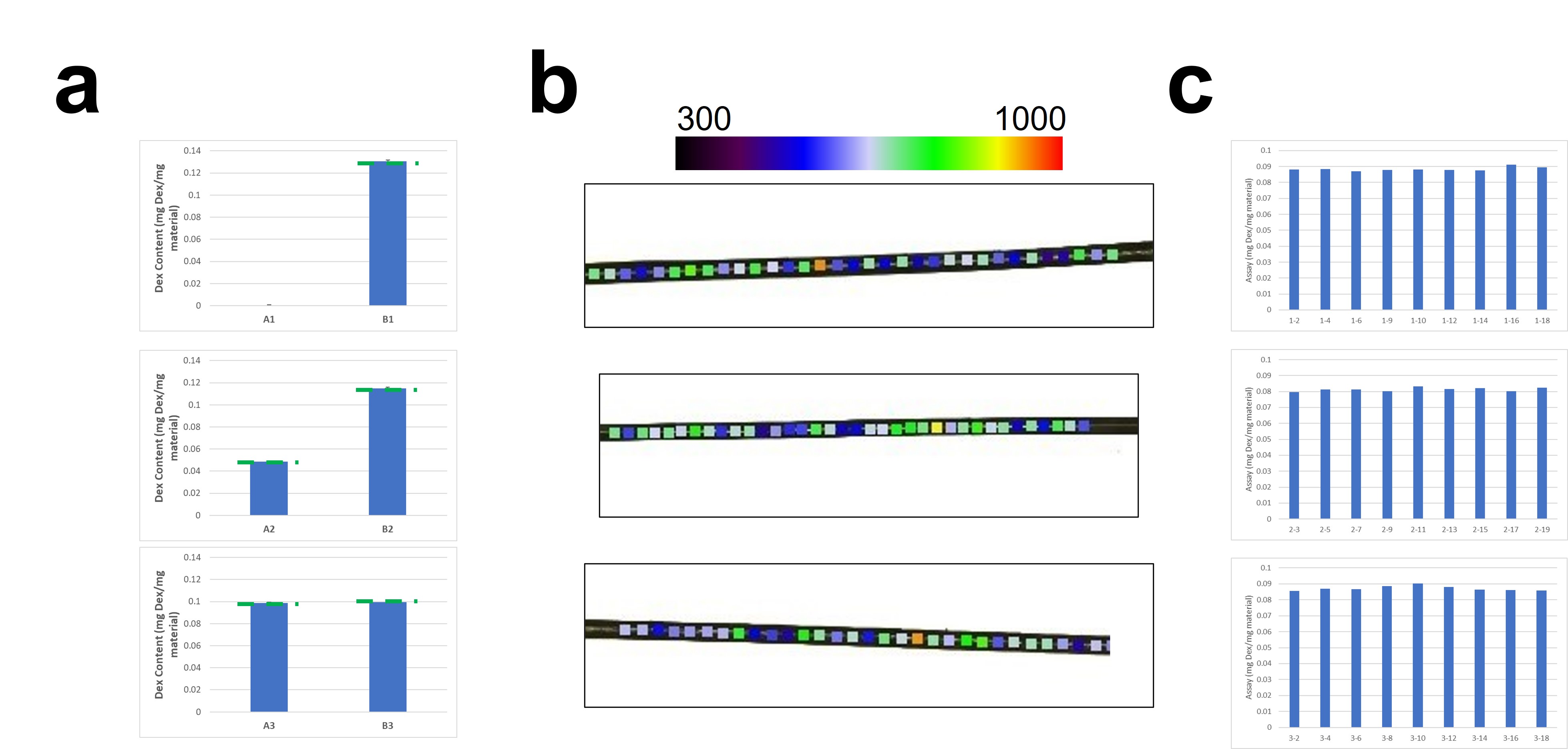
Pre-polymer A and B component extraction efficiencies of three 10% dexamethasone loaded PGSU formulations (a). Backscatter Raman data collected on final PGSU microdevices (b). Assay across the batch for final PGSU microdevices (c).
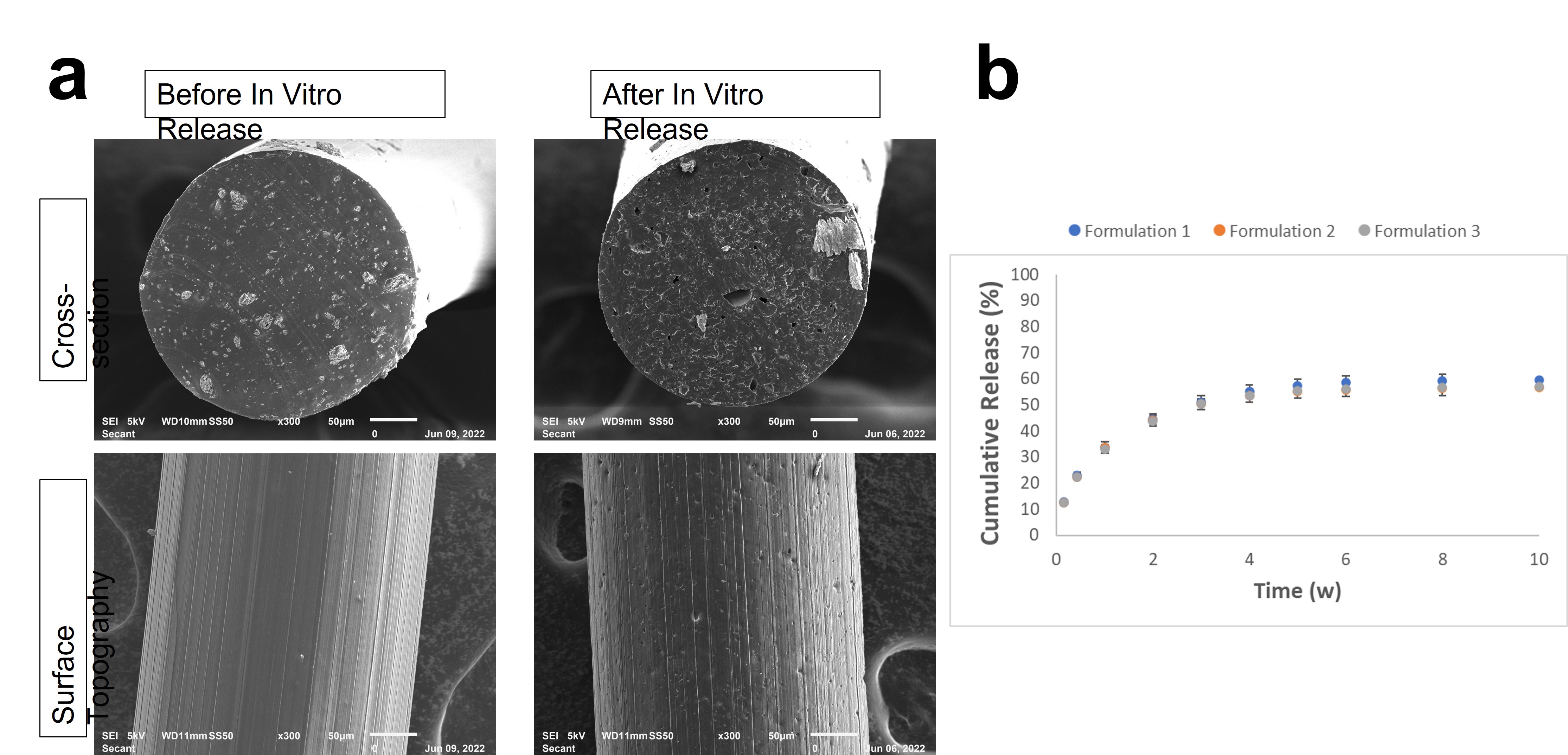
SEM images before and after 10 weeks of in vitro release of 10% dexamethasone loaded PGSU microdevices (a). In vitro release profiles for three 10% dexamethasone loaded PGSU formulations (b).
Formulation and Delivery - Chemical - Drug Delivery
Category: Late Breaking Poster Abstract
(M1530-03-15) Drug-Loaded Hydralese™ Poly(Glycerol Sebacate) Urethane Elastomers as Intra-vitreal Microdevices for Ocular Drug Delivery Applications
Monday, October 17, 2022
3:30 PM – 4:30 PM ET
- SR
Stephanie Reed, Ph.D.
Secant Group
Telford, Pennsylvania, United States - JM
Joshua Mealy, Ph.D.
Secant Group
551 East Church Ave, Pennsylvania, United States
Presenting Author(s)
Main Author(s)
Purpose: Long-acting implantables (LAIs) have become increasingly important in providing novel formulations for active pharmaceutical ingredients (APIs). Traditionally, these systems have utilized polymers such as poly(lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), and ethylene vinyl acetate (EVA). To provide sustained release, these polymers often rely on diffusion-based mechanisms where PLGA and PCL exhibit bulk-eroding degradation that results in a high burst release and less predictable mass loss and drug delivery. Biodegradable poly(glycerol sebacate) urethane--HydraleseTM (PGSU)—is a novel elastomer that may be used for the next generation of LAIs. PGSU is unique in its ability to provide linear release kinetics, high drug loading (often >60% w/w), and a surface-eroding degradation mechanism that minimizes burst release. Furthermore, PGSU maintains excellent mechanical properties such as high flexibility, shelf stability under ambient conditions, and biocompatibility. Ocular therapeutics often find benefit from controlled release dosage forms. Patient compliance is often low with the frequent dosing regimen that is prescribed with many ophthalmic medications (often several times per day for eyedrops, or weekly or monthly injections, depending on ocular disease and indication). Patient tolerability and safety are especially important considerations for repeat dosing. The immune-privileged nature of the eye allows for the direct implantation of a variety of controlled release dosage forms without some of the challenges faced in other areas of the body. From a manufacturing perspective, these intra-ocular devices must be made small enough to be delivered using appropriate gauge needles (often requiring diameters of 450um or less) and must be mechanically robust enough to be handled during drug loading and manufacturing. From a therapeutic perspective, intra-ocular devices must provide sufficient duration of treatment, must not negatively impact vision or physiological function, and must be eventually degradable. The controlled release, non-swelling, flexible, and biocompatible benefits of PGSU, led our group to investigate the development and characterization of PGSU microdevices intended for intra-vitreal administration.
Methods: PGS resin was utilized to manufacture drug-loaded Hydralese (PGSU) microdevices approximately 300 μm in diameter and 10 mm in length. In this study, we investigated a 10% w/w final loading of dexamethasone using multiple different pre-polymer formulations. Dexamethasone was selected as an API for these systems due to its utility in the treatment of various ocular diseases, such as diabetic macular edema and uveitis. A two-component dual-barrel cartridge system was utilized to manufacture microdevices. Process mixing uniformity and final product content uniformity were evaluated using solvent extraction procedures followed by quantification with high performance liquid chromatography (HPLC). To identify spatial content uniformity of samples, a Raman microscope equipped with a 785nm laser was utilized. Microdevices were spatially mapped by gathering spectra at various points along each device length, then analyzed to determine area under the curve for Raman bands associated with dexamethasone. Drug release studies were conducted on n=3 microdevices formulated using 10% w/w dexamethasone loading. Microdevices were incubated in 1mL of 0.1M PBS at 37 °C under gentle agitation. Media was sampled and replaced weekly, and releaseates were quantified for dexamethasone concentration using HPLC. Final microdevice constructs were analyzed post-dissolution using extraction methods described previously and SEM to assess drug release and material degradation.
Results: Microdevices manufactured using this technique could be fabricated at a variety of sizes ranging from as small as 200 μm in diameter (Figure 1) and up to 70% w/w API loading. Three formulations of 10% w/w dexamethasone loading were manufactured at 300 μm diameter. These formulations varied the distribution of API in the A and B component mixes to determine which would result in optimal mixing. In-process samples were taken of A component and B component polymer mixes and assessed for content uniformity. Across 5 samples in each mix, drug loading was assessed to be >98% of theoretical loading with relative standard deviations of < 1.5%, indicating complete and uniform mixing (Figure 2A). Content uniformity of final microdevices assessed via Raman microscopy also showed consistent spatial distribution of API throughout the length of these microdevices (Figure 2B). Finally, extraction of microdevices sampled throughout the manufacturing batch indicated uniform API content at all points during manufacturing. Microdevices of each formulation (n=3) were assayed for in vitro release over 10 weeks. Comparison of pre- and post-release cross-sectional SEM images showed dissolution and release of API particles. Analysis of microdevice surface topographies showed slight pitting after 10 weeks of in vitro release, which indicates early signs of polymer erosion. Notably, no polymer swelling was observed, which is a benefit of the PGSU crosslinked polymer network. Finally, these constructs provided sustained release of dexamethasone for around 8 weeks prior to depletion of payload.
Conclusion: PGSU is a useful polymer excipient in the manufacture of drug-loaded microdevices for use as intra-vitreal implants. Manufacturing processes for PGSU materials support the fabrication of uniform, flexible, and small diameter devices that can sustain the release of dexamethasone for at least 8 weeks in vitro.

Drug loaded PGSU microdevices formed using a variety of diameters.

Pre-polymer A and B component extraction efficiencies of three 10% dexamethasone loaded PGSU formulations (a). Backscatter Raman data collected on final PGSU microdevices (b). Assay across the batch for final PGSU microdevices (c).

SEM images before and after 10 weeks of in vitro release of 10% dexamethasone loaded PGSU microdevices (a). In vitro release profiles for three 10% dexamethasone loaded PGSU formulations (b).
