Back
Purpose: Colistin is a last line antibiotic against multidrug resistant Gram-negative bacteria that infect human lungs. However, its pulmonary administration in high doses causes concentration- and time-dependent toxicity in lung epithelial cells (Ahmed et al., 2017). When used in combination with tobramycin, the toxicity of colistin is reduced (Zhou, Ahmed, Li, & Azad, 2022) and its antibacterial activity is synergistically enhanced (Herrmann et al., 2010; Kashyap, Kaur, Sharma, & Capalash, 2021). We therefore wish to develop dry powder formulations containing both colistin sulfate and tobramycin sulfate. However, if we are to prepare highly effective and stable formulations, it is important to assess the aerosolization performance of these formulations and to determine how it is affected by environmental factors like humidity.
Methods: We manufactured the formulations on a BUCHI B-290 mini spray dryer using 21.7 mg/mL aqueous feed solutions with no excipients. Colistin and tobramycin were combined at 1:1 and 1:5 molar ratios to make the combination formulations. We measured in vitro aerosolization using a Next Generation Impactor; formulation was capsuled and loaded into an RS01 DPI device; about 4 L of air was pumped through the inhaler at 100 L/min for dispersion. Percentage of recovered drug that exited the device is termed the emitted dose (ED). Fine particle fraction (FPF) is the percentage of the total recovered drug with aerodynamic diameter below 5 μm. The emitted fine particle fraction (E-FPF) is calculated as the percentage of emitted particles with an aerodynamic diameter below 5 μm. Particle surface composition was measured using X-ray photoelectron spectroscopy. Inverse gas chromatography was used to estimate dispersive and specific surface energy of formulation particles. Retention times of a series of hydrocarbon vapors passed through a packed column of a formulation were used to calculate dispersive surface energy (Schultz method). Similarly, polar probes were used to determine specific surface energy (Good-van Oss theory). We determined particle size distribution using laser diffraction, imaged particle morphology using scanning electron microscopy, and assessed crystallinity using powder X-ray diffraction. Finally, we studied the storage stability of pure and combination formulations at room temperature and two humidity levels - 20 and 55% RH. Over a storage period of four weeks, we measured changes in particle morphology, in vitro aerosolization and crystallization.
Results: Spray dried pure colistin, pure tobramycin, and both combination formulations (1:1 and 1:5 colistin-tobramycin) were amorphous solids. The volume median diameter, or D50, of all spray dried formulations was approximately 2.2 – 2.5 μm and the span value was 1.7 – 2, which indicates suitability for pulmonary delivery as dry powders. The emitted dose (ED), fine particle fraction (FPF) and emitted fine particle fraction (E-FPF) of tobramycin drug showed by both combination formulations was excellent and significantly higher than that showed by spray dried pure tobramycin (Graphic 1). The combination formulations also showed slightly higher ED, FPF and E-FPF values of colistin drug than the pure colistin formulation, although the difference was not always statistically significant. The particle surfaces of both combination formulations were significantly enriched in colistin molecules. In the 1:1 combination, which contained 69% by wt. colistin overall, the particle surfaces showed 88.6 ± 5.0% colistin content. Similarly, the 1:5 combination showed a surface colistin content of 76.8 ± 1.3%, which was dramatically higher than its overall colistin content (31%). Spray dried pure tobramycin had a higher dispersive or non-polar surface energy than both the combination formulations and pure colistin at all fractional coverages (p/po between 0.05 and 0.40) (Graphic 2). The specific or polar surface energy of pure tobramycin was 51-68% higher at p/po of 0.2 or above. The dispersive and specific surface energy profile for spray dried colistin was similar to the combination formulations. The aerosolization performance of both 1:1 and 1:5 combination formulations remained high and stable for four weeks of storage at 20 and 55% RH. In contrast, pure tobramycin showed significant increase in ED and FPF over four weeks. Based on weekly particle size and imaging data, pure colistin and combination formulations experienced negligible agglomeration during the storage study. However, pure tobramycin particles progressively fused with each other via liquid bridges, which occurred faster and to a greater extent at 55% than 20% RH (Graphic 3). None of the formulations crystallized regardless of the storage condition.
Conclusion: Combination formulations of colistin and tobramycin prepared by spray drying showed excellent in vitro aerosolization. During the stability study at 20 and 55%RH, both 1:1 and 1:5 colistin-tobramycin formulations showed no moisture-driven agglomeration and maintained high aerosolization performance. Colistin molecules were enriched on the surface of combination particles. This appeared to contribute to their lower surface energy and lower affinity to moisture compared to pure spray dried tobramycin. Overall, the excellent aerosolization of the combination formulations makes them highly efficient for pulmonary delivery, and thus, are suitable to treat lung infections caused by multi-drug resistant pathogens.
References: Ahmed, M. U., Velkov, T., Lin, Y.-W., Yun, B., Nowell, C. J., Zhou, F., . . . Li, J. (2017). Potential toxicity of polymyxins in human lung epithelial cells. Antimicrobial agents and chemotherapy, 61(6).
Herrmann, G., Yang, L., Wu, H., Song, Z., Wang, H., Høiby, N., . . . Döring, G. (2010). Colistin-Tobramycin Combinations Are Superior to Monotherapy Concerning the Killing of Biofilm Pseudomonas aeruginosa. The Journal of Infectious Diseases, 202(10), 1585-1592. doi:10.1086/656788
Kashyap, S., Kaur, S., Sharma, P., & Capalash, N. (2021). Combination of colistin and tobramycin inhibits persistence of Acinetobacter baumannii by membrane hyperpolarization and down-regulation of efflux pumps. Microbes and Infection, 104795. doi:10.1016/j.micinf.2021.104795
Zhou, Q., Ahmed, M. U., Li, J., & Azad, M. A. K. (2022). United States of America Patent No. US2021030393.
Acknowledgments: This research project was supported by the National Institute of Allergy and Infectious Diseases of the National Institute of Health under Award Number R01AI132681 and R01AI146160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.L. is an Australian National Health and Medical Research Council (NHMRC) Principal Research Fellow (APP1157909). Authors have no conflict of interest on this work.
Graphic 1: Emitted dose, fine particle fraction, and emitted fine particle fraction of the freshly spray dried combination formulations of colistin and tobramycin (mean ± S.D., n=3 or 4)
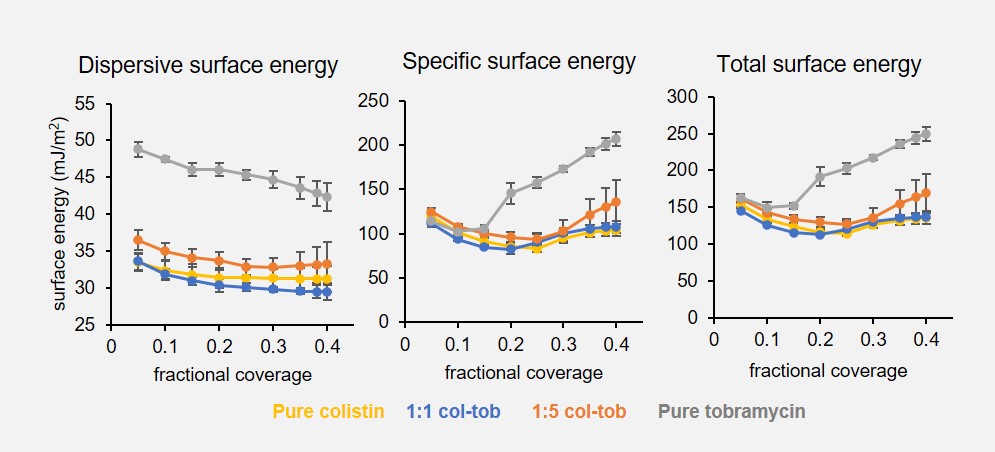
Graphic 2: Dispersive, specific, and total surface energy of spray dried formulations
Graphic 3: Morphology of fresh pure colistin and combination formulations, which remained unchanged after storage study. Agglomeration of pure tobramycin formulation particles after four weeks at 20 and 55%RH
Formulation and Delivery - Chemical - Formulation
Category: Late Breaking Poster Abstract
(M1530-07-41) Spray Dried Combination Formulations of Tobramycin and Colistin for Pulmonary Delivery Show Excellent In Vitro Powder Aerosolization
Monday, October 17, 2022
3:30 PM – 4:30 PM ET
- VP
Vaibhav Pathak, MS
Purdue University
West Lafayette, Indiana, United States - VP
Vaibhav Pathak, MS
Purdue University
West Lafayette, Indiana, United States
Presenting Author(s)
Main Author(s)
Purpose: Colistin is a last line antibiotic against multidrug resistant Gram-negative bacteria that infect human lungs. However, its pulmonary administration in high doses causes concentration- and time-dependent toxicity in lung epithelial cells (Ahmed et al., 2017). When used in combination with tobramycin, the toxicity of colistin is reduced (Zhou, Ahmed, Li, & Azad, 2022) and its antibacterial activity is synergistically enhanced (Herrmann et al., 2010; Kashyap, Kaur, Sharma, & Capalash, 2021). We therefore wish to develop dry powder formulations containing both colistin sulfate and tobramycin sulfate. However, if we are to prepare highly effective and stable formulations, it is important to assess the aerosolization performance of these formulations and to determine how it is affected by environmental factors like humidity.
Methods: We manufactured the formulations on a BUCHI B-290 mini spray dryer using 21.7 mg/mL aqueous feed solutions with no excipients. Colistin and tobramycin were combined at 1:1 and 1:5 molar ratios to make the combination formulations. We measured in vitro aerosolization using a Next Generation Impactor; formulation was capsuled and loaded into an RS01 DPI device; about 4 L of air was pumped through the inhaler at 100 L/min for dispersion. Percentage of recovered drug that exited the device is termed the emitted dose (ED). Fine particle fraction (FPF) is the percentage of the total recovered drug with aerodynamic diameter below 5 μm. The emitted fine particle fraction (E-FPF) is calculated as the percentage of emitted particles with an aerodynamic diameter below 5 μm. Particle surface composition was measured using X-ray photoelectron spectroscopy. Inverse gas chromatography was used to estimate dispersive and specific surface energy of formulation particles. Retention times of a series of hydrocarbon vapors passed through a packed column of a formulation were used to calculate dispersive surface energy (Schultz method). Similarly, polar probes were used to determine specific surface energy (Good-van Oss theory). We determined particle size distribution using laser diffraction, imaged particle morphology using scanning electron microscopy, and assessed crystallinity using powder X-ray diffraction. Finally, we studied the storage stability of pure and combination formulations at room temperature and two humidity levels - 20 and 55% RH. Over a storage period of four weeks, we measured changes in particle morphology, in vitro aerosolization and crystallization.
Results: Spray dried pure colistin, pure tobramycin, and both combination formulations (1:1 and 1:5 colistin-tobramycin) were amorphous solids. The volume median diameter, or D50, of all spray dried formulations was approximately 2.2 – 2.5 μm and the span value was 1.7 – 2, which indicates suitability for pulmonary delivery as dry powders. The emitted dose (ED), fine particle fraction (FPF) and emitted fine particle fraction (E-FPF) of tobramycin drug showed by both combination formulations was excellent and significantly higher than that showed by spray dried pure tobramycin (Graphic 1). The combination formulations also showed slightly higher ED, FPF and E-FPF values of colistin drug than the pure colistin formulation, although the difference was not always statistically significant. The particle surfaces of both combination formulations were significantly enriched in colistin molecules. In the 1:1 combination, which contained 69% by wt. colistin overall, the particle surfaces showed 88.6 ± 5.0% colistin content. Similarly, the 1:5 combination showed a surface colistin content of 76.8 ± 1.3%, which was dramatically higher than its overall colistin content (31%). Spray dried pure tobramycin had a higher dispersive or non-polar surface energy than both the combination formulations and pure colistin at all fractional coverages (p/po between 0.05 and 0.40) (Graphic 2). The specific or polar surface energy of pure tobramycin was 51-68% higher at p/po of 0.2 or above. The dispersive and specific surface energy profile for spray dried colistin was similar to the combination formulations. The aerosolization performance of both 1:1 and 1:5 combination formulations remained high and stable for four weeks of storage at 20 and 55% RH. In contrast, pure tobramycin showed significant increase in ED and FPF over four weeks. Based on weekly particle size and imaging data, pure colistin and combination formulations experienced negligible agglomeration during the storage study. However, pure tobramycin particles progressively fused with each other via liquid bridges, which occurred faster and to a greater extent at 55% than 20% RH (Graphic 3). None of the formulations crystallized regardless of the storage condition.
Conclusion: Combination formulations of colistin and tobramycin prepared by spray drying showed excellent in vitro aerosolization. During the stability study at 20 and 55%RH, both 1:1 and 1:5 colistin-tobramycin formulations showed no moisture-driven agglomeration and maintained high aerosolization performance. Colistin molecules were enriched on the surface of combination particles. This appeared to contribute to their lower surface energy and lower affinity to moisture compared to pure spray dried tobramycin. Overall, the excellent aerosolization of the combination formulations makes them highly efficient for pulmonary delivery, and thus, are suitable to treat lung infections caused by multi-drug resistant pathogens.
References: Ahmed, M. U., Velkov, T., Lin, Y.-W., Yun, B., Nowell, C. J., Zhou, F., . . . Li, J. (2017). Potential toxicity of polymyxins in human lung epithelial cells. Antimicrobial agents and chemotherapy, 61(6).
Herrmann, G., Yang, L., Wu, H., Song, Z., Wang, H., Høiby, N., . . . Döring, G. (2010). Colistin-Tobramycin Combinations Are Superior to Monotherapy Concerning the Killing of Biofilm Pseudomonas aeruginosa. The Journal of Infectious Diseases, 202(10), 1585-1592. doi:10.1086/656788
Kashyap, S., Kaur, S., Sharma, P., & Capalash, N. (2021). Combination of colistin and tobramycin inhibits persistence of Acinetobacter baumannii by membrane hyperpolarization and down-regulation of efflux pumps. Microbes and Infection, 104795. doi:10.1016/j.micinf.2021.104795
Zhou, Q., Ahmed, M. U., Li, J., & Azad, M. A. K. (2022). United States of America Patent No. US2021030393.
Acknowledgments: This research project was supported by the National Institute of Allergy and Infectious Diseases of the National Institute of Health under Award Number R01AI132681 and R01AI146160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. J.L. is an Australian National Health and Medical Research Council (NHMRC) Principal Research Fellow (APP1157909). Authors have no conflict of interest on this work.

Graphic 1: Emitted dose, fine particle fraction, and emitted fine particle fraction of the freshly spray dried combination formulations of colistin and tobramycin (mean ± S.D., n=3 or 4)

Graphic 2: Dispersive, specific, and total surface energy of spray dried formulations

Graphic 3: Morphology of fresh pure colistin and combination formulations, which remained unchanged after storage study. Agglomeration of pure tobramycin formulation particles after four weeks at 20 and 55%RH
