Back
Purpose: Cyanide is widely encountered in society as it is used in several different industries, is released through the burning of materials, and is contained in many of the seeds of fruit that we eat. Of significant concern is cyanide exposure through malicious misuse. Development of cyanide countermeasures to mitigate acute lethal exposures require fast administration and rapid chemical scavenging, properties not inherent in the currently approved agents. Previous work using platinum-DMSO complexes as scavengers were demonstrated to be effective at mitigating cyanide toxicity in zebrafish, mice, rabbits and pigs.1,2 These complexes were suspected to be the active form, where it was hypothesized that the trans effect from the DMSO sulfide interaction with platinum led to the rapid cyanide scavenging.3–6 Investigating the trans effect, an additional 6 bidentate amino-thioether (e.g. methionine) platinum agents were generated and demonstrated to possess cyanide scavenging in vitro and efficacy against cyanide poisoning in vivo. Work here focuses on in vitro characterization of these platinum-sulfur complexes and the reactions with cyanide. These agents represent a new hit-to-lead candidate series for development of next generation cyanide countermeasures.
Methods: An Epsilon 4 X-Ray Fluorescence spectrometer with a silver anode X-ray tube was used to determine the platinum composition in each complex. Briefly, X-ray fluorescence spectra of platinum complexes were determined using manganese chloride as an internal standard. The weight percent platinum (% w/w) was obtained for each complex to normalize for platinum content in each batch of material. HPLC analysis was performed on an Agilent 1100 equipped with a Raptor PolarX 50 mm x 2.1 mm column to separate reaction mixtures of platinum. All reactions with platinum and cyanide were performed in pH 7.5 phosphate buffer. Cyanide was added to the platinum samples at 1x, 2x, 4x, 7x, 10x molar equivalences (n=3 for each preparation). Platinum-cyanide complexes were quantified at 260 nm with a UV-DAD. Normalization of titration samples was performed to prioritize complexes based on their ability to produce Pt(CN)42-. A Cary60 (Agilent) was used for all the UV-Vis experiments to monitor the reaction between platinum and cyanide. All cyanide reactions were performed in phosphate buffer using 40x molar excess cyanide to platinum. Data collection was performed with a scan speed of 24,000nm/sec between 300-220 nm and determined over a time-period of 300 minutes. Kinetic data were then fit using a one-phase exponential curve to derive apparent rate constants. 195Pt spectra were acquired using a Bruker DRX 500 MHz spectrometer to confirm the identity and assess the ability to form Pt(CN)42- of each Pt-complex. 1H and 13C spectra were acquired using the same spectrometer or a Bruker Avance 800 MHz spectrometer. Initial in vivo platinum-cyanide reactivity and survival was assessed in an established zebrafish assay Briefly, zebrafish larvae were plated into 96-well plates in HEPEs buffered Tubingen E3 medium (n=5 per well). After 6 days, the zebrafish were dosed with 50 µM cyanide and each platinum complex (1-250 µM) to obtain a dose-survival response, with survival documented after 4 h of exposure post-treatment. Complexes were rank ordered and then investigated in a murine lethal cyanide inhalation model to determine the ability of each complex to rescue mice. Here, mice were placed in a gastight chamber and exposed to a lethal concentration of cyanide gas for 15 minutes. After 15 minutes the mouse was removed, injected with a specific complex, and placed back into the cyanide chamber for an additional 25 minutes. Survival at the end of the assay was recorded and equated to an efficacious response.
Results: Platinum leads were tested in vitro using multiple assays (e.g., UV-Vis, HPLC and NMR) to confirm reactions with cyanide. Results show consumption of cyanide occurred through chelation to form platinum-cyanide complexes. Reactions under first-order conditions for the amino-thioether bidentate complexes 6, 10 and 11 to form Pt(CN)42- with Kobs >15 min-1. Slower complexes 2, 7, 8 and 9 having Kobs 0.2-0.6 min-1. Pt-DMSO complexes had Kobs < 0.03 min-1 (Table 1). Dominate species in solution was Pt(CN)42- as was identified in HPLC and NMR (Figure <2). Platinum thioether complexes demonstrated higher efficacy than the Pt-DMSO and Pt-chloride complexes that were studied previously. The zebrafish cyanide model resulted in 1.2 µg Pt/mL for complex 6 versus Pt-DMSO complexes at 3.3 µg Pt/mL. Complex 6 also had 100% survival at 17 mg Pt/kg using the lethal mouse inhalation model. This contrasts with the previously most efficacious Pt-DMSO complex requiring 49 mg Pt/kg for 100% rescue in this model.
Conclusion: The work here reveals that our platinum-based agent scavenging can be altered based on the amino-sulfur ligands to modulate scavenging cyanide effectiveness. These agents represent a new class of compounds that may be useful for further development as cyanide scavengers with potential utility in a mass casualty setting where intramuscular injection may be used.
References: 1. Morningstar, J. et al. Intramuscular administration of hexachloroplatinate reverses cyanide-induced metabolic derangements and counteracts severe cyanide poisoning. FASEB BioAdvances 1, 81–92 (2019).
2. Nath, A. K. et al. Cisplatin Analogs Confer Protection against Cyanide Poisoning. Cell Chem. Biol. 24, 565-575.e4 (2017).
3. Bose, R. N., Ghosh, S. K. & Moghaddas, S. Kinetic analysis of the cis-diamminedichloroplatinum(II)-cysteine reaction: Implications to the extent of platinum-DNA binding. J. Inorg. Biochem. 65, 199–205 (1997).
4. Chen, S. et al. Stability, Reduction, and Cytotoxicity of Platinum(IV) Anticancer Prodrugs Bearing Carbamate Axial Ligands: Comparison with Their Carboxylate Analogues. Inorg. Chem. 59, 11676–11687 (2020).
5. Guyon, F., Knorr, M., Garillon, A. & Strohmann, C. Platinum(II) Complexes Bearing a Thiolate/Thioether Ligand – Hemilability vs. Dealkylation. Eur. J. Inorg. Chem. 2012, 282–291 (2012).
6. Ma, G. et al. The reaction of a platinated methionine motif of CTR1 with cysteine and histidine is dependent upon the type of precursor platinum complex. J. Inorg. Biochem. 153, 239–246 (2015).
Acknowledgments:
Funding: NINDS-CounterAct Program (U54 NS112107) “Discovery and Development of Novel Classes of Cyanide Countermeasure.” Graduate Assistance in Areas of National Need (GAANN) CFDA Number: 84.200
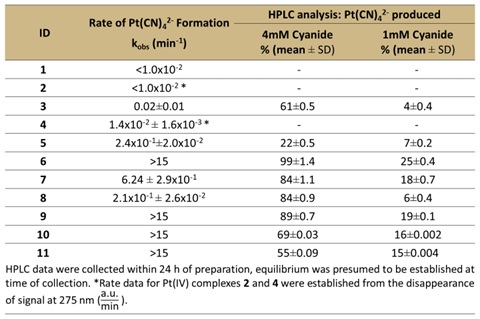
1-2 are Pt-chloride complexes, 3-5 are Pt-DMSO complexes and 6-11 are new bidentate amino thioether ligands on platinum identified in this work. Analysis of platinum-based cyanide scavengers by UV-Vis in first order conditions with excess cyanide. Data shows that complexes 6, 9, 10 and 11 were the fastest of the 11 tested in this work. Additionally, HPLC analysis shows that complexes 6 and 9 produce the most tetracyanoplatinate under the conditions tested.
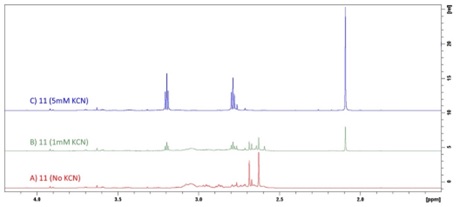
Pt amine-sulfide complex 11 reaction with KCN was monitored by 1H NMR at 298K. A) 1 mM compound 11 alone in 50 mM phosphate, pH 7.5, 10% D2O; B) with 1 mM KCN added; C) with 5 mM (final concentration) KCN added.
Formulation and Delivery - Chemical - Formulation
Category: Late Breaking Poster Abstract
(M1130-07-41) Optimization of Novel Platinum Based Cyanide Scavengers: In Vitro Prioritization
Monday, October 17, 2022
11:30 AM – 12:30 PM ET
- MB
Matthew Behymer
Purdue University
West Lafayette, Indiana, United States - MB
Matthew Behymer
Purdue University
West Lafayette, Indiana, United States
Presenting Author(s)
Main Author(s)
Purpose: Cyanide is widely encountered in society as it is used in several different industries, is released through the burning of materials, and is contained in many of the seeds of fruit that we eat. Of significant concern is cyanide exposure through malicious misuse. Development of cyanide countermeasures to mitigate acute lethal exposures require fast administration and rapid chemical scavenging, properties not inherent in the currently approved agents. Previous work using platinum-DMSO complexes as scavengers were demonstrated to be effective at mitigating cyanide toxicity in zebrafish, mice, rabbits and pigs.1,2 These complexes were suspected to be the active form, where it was hypothesized that the trans effect from the DMSO sulfide interaction with platinum led to the rapid cyanide scavenging.3–6 Investigating the trans effect, an additional 6 bidentate amino-thioether (e.g. methionine) platinum agents were generated and demonstrated to possess cyanide scavenging in vitro and efficacy against cyanide poisoning in vivo. Work here focuses on in vitro characterization of these platinum-sulfur complexes and the reactions with cyanide. These agents represent a new hit-to-lead candidate series for development of next generation cyanide countermeasures.
Methods: An Epsilon 4 X-Ray Fluorescence spectrometer with a silver anode X-ray tube was used to determine the platinum composition in each complex. Briefly, X-ray fluorescence spectra of platinum complexes were determined using manganese chloride as an internal standard. The weight percent platinum (% w/w) was obtained for each complex to normalize for platinum content in each batch of material. HPLC analysis was performed on an Agilent 1100 equipped with a Raptor PolarX 50 mm x 2.1 mm column to separate reaction mixtures of platinum. All reactions with platinum and cyanide were performed in pH 7.5 phosphate buffer. Cyanide was added to the platinum samples at 1x, 2x, 4x, 7x, 10x molar equivalences (n=3 for each preparation). Platinum-cyanide complexes were quantified at 260 nm with a UV-DAD. Normalization of titration samples was performed to prioritize complexes based on their ability to produce Pt(CN)42-. A Cary60 (Agilent) was used for all the UV-Vis experiments to monitor the reaction between platinum and cyanide. All cyanide reactions were performed in phosphate buffer using 40x molar excess cyanide to platinum. Data collection was performed with a scan speed of 24,000nm/sec between 300-220 nm and determined over a time-period of 300 minutes. Kinetic data were then fit using a one-phase exponential curve to derive apparent rate constants. 195Pt spectra were acquired using a Bruker DRX 500 MHz spectrometer to confirm the identity and assess the ability to form Pt(CN)42- of each Pt-complex. 1H and 13C spectra were acquired using the same spectrometer or a Bruker Avance 800 MHz spectrometer. Initial in vivo platinum-cyanide reactivity and survival was assessed in an established zebrafish assay Briefly, zebrafish larvae were plated into 96-well plates in HEPEs buffered Tubingen E3 medium (n=5 per well). After 6 days, the zebrafish were dosed with 50 µM cyanide and each platinum complex (1-250 µM) to obtain a dose-survival response, with survival documented after 4 h of exposure post-treatment. Complexes were rank ordered and then investigated in a murine lethal cyanide inhalation model to determine the ability of each complex to rescue mice. Here, mice were placed in a gastight chamber and exposed to a lethal concentration of cyanide gas for 15 minutes. After 15 minutes the mouse was removed, injected with a specific complex, and placed back into the cyanide chamber for an additional 25 minutes. Survival at the end of the assay was recorded and equated to an efficacious response.
Results: Platinum leads were tested in vitro using multiple assays (e.g., UV-Vis, HPLC and NMR) to confirm reactions with cyanide. Results show consumption of cyanide occurred through chelation to form platinum-cyanide complexes. Reactions under first-order conditions for the amino-thioether bidentate complexes 6, 10 and 11 to form Pt(CN)42- with Kobs >15 min-1. Slower complexes 2, 7, 8 and 9 having Kobs 0.2-0.6 min-1. Pt-DMSO complexes had Kobs < 0.03 min-1 (Table 1). Dominate species in solution was Pt(CN)42- as was identified in HPLC and NMR (Figure <2). Platinum thioether complexes demonstrated higher efficacy than the Pt-DMSO and Pt-chloride complexes that were studied previously. The zebrafish cyanide model resulted in 1.2 µg Pt/mL for complex 6 versus Pt-DMSO complexes at 3.3 µg Pt/mL. Complex 6 also had 100% survival at 17 mg Pt/kg using the lethal mouse inhalation model. This contrasts with the previously most efficacious Pt-DMSO complex requiring 49 mg Pt/kg for 100% rescue in this model.
Conclusion: The work here reveals that our platinum-based agent scavenging can be altered based on the amino-sulfur ligands to modulate scavenging cyanide effectiveness. These agents represent a new class of compounds that may be useful for further development as cyanide scavengers with potential utility in a mass casualty setting where intramuscular injection may be used.
References: 1. Morningstar, J. et al. Intramuscular administration of hexachloroplatinate reverses cyanide-induced metabolic derangements and counteracts severe cyanide poisoning. FASEB BioAdvances 1, 81–92 (2019).
2. Nath, A. K. et al. Cisplatin Analogs Confer Protection against Cyanide Poisoning. Cell Chem. Biol. 24, 565-575.e4 (2017).
3. Bose, R. N., Ghosh, S. K. & Moghaddas, S. Kinetic analysis of the cis-diamminedichloroplatinum(II)-cysteine reaction: Implications to the extent of platinum-DNA binding. J. Inorg. Biochem. 65, 199–205 (1997).
4. Chen, S. et al. Stability, Reduction, and Cytotoxicity of Platinum(IV) Anticancer Prodrugs Bearing Carbamate Axial Ligands: Comparison with Their Carboxylate Analogues. Inorg. Chem. 59, 11676–11687 (2020).
5. Guyon, F., Knorr, M., Garillon, A. & Strohmann, C. Platinum(II) Complexes Bearing a Thiolate/Thioether Ligand – Hemilability vs. Dealkylation. Eur. J. Inorg. Chem. 2012, 282–291 (2012).
6. Ma, G. et al. The reaction of a platinated methionine motif of CTR1 with cysteine and histidine is dependent upon the type of precursor platinum complex. J. Inorg. Biochem. 153, 239–246 (2015).
Acknowledgments:
Funding: NINDS-CounterAct Program (U54 NS112107) “Discovery and Development of Novel Classes of Cyanide Countermeasure.” Graduate Assistance in Areas of National Need (GAANN) CFDA Number: 84.200

1-2 are Pt-chloride complexes, 3-5 are Pt-DMSO complexes and 6-11 are new bidentate amino thioether ligands on platinum identified in this work. Analysis of platinum-based cyanide scavengers by UV-Vis in first order conditions with excess cyanide. Data shows that complexes 6, 9, 10 and 11 were the fastest of the 11 tested in this work. Additionally, HPLC analysis shows that complexes 6 and 9 produce the most tetracyanoplatinate under the conditions tested.

Pt amine-sulfide complex 11 reaction with KCN was monitored by 1H NMR at 298K. A) 1 mM compound 11 alone in 50 mM phosphate, pH 7.5, 10% D2O; B) with 1 mM KCN added; C) with 5 mM (final concentration) KCN added.
