Back
Purpose: About 90% of the new chemical entities in the discovery pipeline and 40% of the market approved drugs are poorly water soluble and these solubility issues hinder their development as oral therapeutic agents. The intrinsic dissolution rate (IDR) studies will help to assess the developability of these compounds into potential products. The present study focuses on evaluating the solubility behavior of sulfamethoxazole (SMZ) and its amorphous solid dispersions (ASDs) using equilibrium solubility and intrinsic dissolution studies.
Methods: Preparation of sulfamethoxazole ASDs - The ASDs and physical mixtures of SMZ were prepared in varying ratios of Polyvinyl pyrrolidone K29/32 polymer (1:1,1:5,1:10 w/w). Similarly, physical mixtures and ASDs of SMZ and polyvinyl pyrrolidone-co vinyl acetate were also prepared at the same ratios. Equilibrium aqueous solubilities of the prepared compositions of physical mixtures and ASDs equilibrium aqueous solubility was determined. Sulfamethoxazole ASDs were prepared by Hot melt method using polyvinyl pyrrolidone (PVP) and poly vinyl pyrrolidone co vinyl acetate (PVP/VA) polymers. The ASDs were prepared by mixing the weighed amount of the API and polymer and were mixed and heated in a porcelain crucible until molten. This molten mass was cooled and pulverized using mortar and pestle and passed through sieve with 40 number mesh. Physical mixtures were prepared by mixing the API and the polymer and grinding in mortar with pestle. This mixture was sieved through a 40 number mesh to obtain uniform particle size. Characterization by Differential scanning calorimetry (DSC)- Both the ASDs and physical mixtures of SMZ were characterized by DSC using aluminum pans at a temperature ramping of 10ºC per minute in nitrogen atmosphere. All the samples were analyzed over the temperature range of 25 ºC - 500 ºC. Equilibrium aqueous solubility- The equilibrium aqueous solubility of the pure API, physical mixtures and the ASDs of SMZ were determined using Shake flask method. Weighed amount of each physical mixtures and ASDs containing an equivalent amount of API were added to glass vials and 5mL of water was added. The vials were vortexed for 30 seconds to disperse the contents and were set for shaking at 25ºC for 24hours. Later, 0.5mL of sample was aliquoted and transferred to a centrifuge tube with 0.45µm cellulose acetate filter and were centrifuged at room temperature for 5 mins at 14000 rpm. Once centrifuged 0.1 mL of the filtrate was drawn and 0.9mL of water was added and were analyzed for the API content by HPLC. Intrinsic dissolution rate (IDR) studies-IDR studies were conducted using rotating disk system. This rotating disk has a die and punch to compress the sample and the die is fixed to the shaft. The dissolution medium was 0.1N Hydrochloric acid. The speed maintained was 75 rpm. Dissolution media samples were collected until 10% of the API in the die was released. The cumulative amount of the API released was plotted against time. The slope of the regression equation gives the dissolution rate in µg.min-1.cm-2. The compositions that exhibited significant enhancement in solubility were chosen for the IDR studies to observe whether the same trend as in equilibrium solubility was maintained.
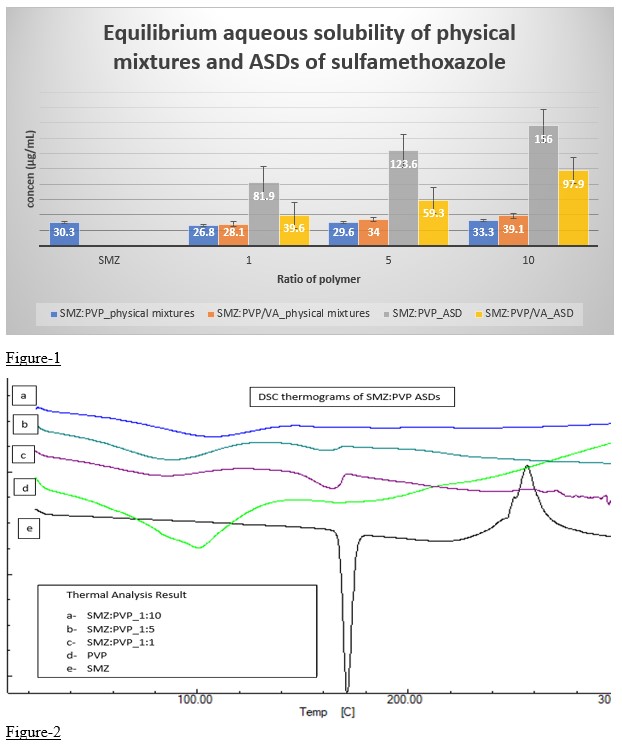
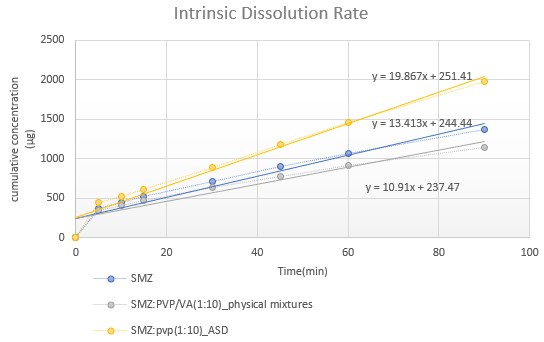
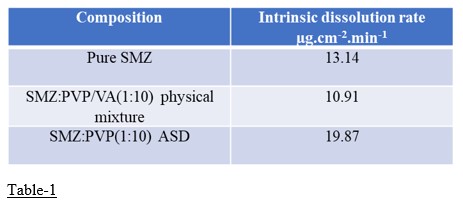
Results: The ADSs of SMZ:PVP(1:10w/w) has exhibited 5-fold increase in equilibrium aqueous solubility when compared to pure SMZ (Fig-1). The P-value obtained by two tailed paired t-test was 0.0001, which shown to be statistically significant. The DSC data showed the amorphous nature of the SMZ (Fig-2) in SMZ:PVP (1:10) ASDs. The intrinsic dissolution rate of SMZ:PVP(1:10 w/w) was 19.87 µg.cm-2.min-1 when compared with 13.41 µg.cm-2.min-1 for pure SMZ. The slope of the linear regression equation of the cumulative concentration vs time graph represents the intrinsic dissolution rate (Fig-3).
Conclusion: The ASDs of SMZ:PVP have exhibited significant increase in the equilibrium solubility, whereas the ASDS of SMZ prepared with PVP/VA did not show any improvement. This improvement in the solubility enhancement is due to the amorphous nature of the SMZ in the ASDs. There has been improvement in the intrinsic dissolution rate of ASD when compared to pure SMZ.
References: 1. Baghel S, Cathcart H, O'Reilly NJ. Polymeric amorphous solid dispersions: a review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. Journal of pharmaceutical sciences. 2016 Sep 1;105(9):2527-44.
2. Sareen S, Mathew G, Joseph L. Improvement in solubility of poor water-soluble drugs by solid dispersion. International journal of pharmaceutical investigation. 2012 Jan;2(1):12.
Formulation and Delivery - Chemical - Biopharmaceutics
Category: Late Breaking Poster Abstract
(M1030-04-20) Amorphous Solid Dispersions for Enhancement of Equilibrium Aqueous Solubility of Sulfamethoxazole
Monday, October 17, 2022
10:30 AM – 11:30 AM ET
- SR
Sridivya Raparla
University of the Pacific
Tracy, California, United States - SR
Sridivya Raparla
University of the Pacific
Tracy, California, United States
Presenting Author(s)
Main Author(s)
Purpose: About 90% of the new chemical entities in the discovery pipeline and 40% of the market approved drugs are poorly water soluble and these solubility issues hinder their development as oral therapeutic agents. The intrinsic dissolution rate (IDR) studies will help to assess the developability of these compounds into potential products. The present study focuses on evaluating the solubility behavior of sulfamethoxazole (SMZ) and its amorphous solid dispersions (ASDs) using equilibrium solubility and intrinsic dissolution studies.
Methods: Preparation of sulfamethoxazole ASDs - The ASDs and physical mixtures of SMZ were prepared in varying ratios of Polyvinyl pyrrolidone K29/32 polymer (1:1,1:5,1:10 w/w). Similarly, physical mixtures and ASDs of SMZ and polyvinyl pyrrolidone-co vinyl acetate were also prepared at the same ratios. Equilibrium aqueous solubilities of the prepared compositions of physical mixtures and ASDs equilibrium aqueous solubility was determined. Sulfamethoxazole ASDs were prepared by Hot melt method using polyvinyl pyrrolidone (PVP) and poly vinyl pyrrolidone co vinyl acetate (PVP/VA) polymers. The ASDs were prepared by mixing the weighed amount of the API and polymer and were mixed and heated in a porcelain crucible until molten. This molten mass was cooled and pulverized using mortar and pestle and passed through sieve with 40 number mesh. Physical mixtures were prepared by mixing the API and the polymer and grinding in mortar with pestle. This mixture was sieved through a 40 number mesh to obtain uniform particle size. Characterization by Differential scanning calorimetry (DSC)- Both the ASDs and physical mixtures of SMZ were characterized by DSC using aluminum pans at a temperature ramping of 10ºC per minute in nitrogen atmosphere. All the samples were analyzed over the temperature range of 25 ºC - 500 ºC. Equilibrium aqueous solubility- The equilibrium aqueous solubility of the pure API, physical mixtures and the ASDs of SMZ were determined using Shake flask method. Weighed amount of each physical mixtures and ASDs containing an equivalent amount of API were added to glass vials and 5mL of water was added. The vials were vortexed for 30 seconds to disperse the contents and were set for shaking at 25ºC for 24hours. Later, 0.5mL of sample was aliquoted and transferred to a centrifuge tube with 0.45µm cellulose acetate filter and were centrifuged at room temperature for 5 mins at 14000 rpm. Once centrifuged 0.1 mL of the filtrate was drawn and 0.9mL of water was added and were analyzed for the API content by HPLC. Intrinsic dissolution rate (IDR) studies-IDR studies were conducted using rotating disk system. This rotating disk has a die and punch to compress the sample and the die is fixed to the shaft. The dissolution medium was 0.1N Hydrochloric acid. The speed maintained was 75 rpm. Dissolution media samples were collected until 10% of the API in the die was released. The cumulative amount of the API released was plotted against time. The slope of the regression equation gives the dissolution rate in µg.min-1.cm-2. The compositions that exhibited significant enhancement in solubility were chosen for the IDR studies to observe whether the same trend as in equilibrium solubility was maintained.
Results: The ADSs of SMZ:PVP(1:10w/w) has exhibited 5-fold increase in equilibrium aqueous solubility when compared to pure SMZ (Fig-1). The P-value obtained by two tailed paired t-test was 0.0001, which shown to be statistically significant. The DSC data showed the amorphous nature of the SMZ (Fig-2) in SMZ:PVP (1:10) ASDs. The intrinsic dissolution rate of SMZ:PVP(1:10 w/w) was 19.87 µg.cm-2.min-1 when compared with 13.41 µg.cm-2.min-1 for pure SMZ. The slope of the linear regression equation of the cumulative concentration vs time graph represents the intrinsic dissolution rate (Fig-3).
Conclusion: The ASDs of SMZ:PVP have exhibited significant increase in the equilibrium solubility, whereas the ASDS of SMZ prepared with PVP/VA did not show any improvement. This improvement in the solubility enhancement is due to the amorphous nature of the SMZ in the ASDs. There has been improvement in the intrinsic dissolution rate of ASD when compared to pure SMZ.
References: 1. Baghel S, Cathcart H, O'Reilly NJ. Polymeric amorphous solid dispersions: a review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. Journal of pharmaceutical sciences. 2016 Sep 1;105(9):2527-44.
2. Sareen S, Mathew G, Joseph L. Improvement in solubility of poor water-soluble drugs by solid dispersion. International journal of pharmaceutical investigation. 2012 Jan;2(1):12.



