Back
Purpose: Ischemia results from an array of clinical pathologies, from organ failure to chronic inflammation. In transplantation, graft endothelial cells’ structures shift in ischemic environments, leading to disruption of the endothelial wall and increased susceptibility to oxidative damage. Management of this universal hypoxic state during preservation would be a starting point to achieve better outcomes in transplant patients. Perfluorocarbons have undergone decades of development as oxygen carriers in biomedical research. They are attractive owing to the absence of infectious risk, existence of commercial manufacturing capabilities, and biological/chemical inertness1. Despite the promising pre-clinical results, there has been limited success translating perfluorocarbon-based oxygen carriers. We have taken a more rigorous approach to develop stable and pharmacologically active perfluorocarbon nanoemulsions (PFC-NEs) as oxygen carriers. Colloidal stability optimization has been previously reported2. Here, the focus is on extending our understanding of the oxygen delivery aspect of PFC-NEs.
Methods: Previously reported PFC-NEs2 served as model formulations. These were prepared in a high-energy microfluidization process and were characterized with dynamic light scattering. In vitro molecular oxygen release was evaluated by observing oxygen replenishment in a flask of deoxygenated release medium after injection of oxygenated PFC-NE into the flask. Dissolved oxygen levels were monitored using a polarographic dissolved oxygen sensor and the results were compared with a fiber optic sensor. RAW 264.7 murine macrophages were cultured in a hypoxic environment using nBIONIX hypoxic culture kits. Image-iT hypoxia green reagent, which has fluorescent properties dependent on oxygen concentration, was used as a proxy of oxygen partial pressure in the intracellular environment. Flow cytometry was utilized to evaluate macrophages labeled with hypoxia green reagent.
Results: The resulting average droplet diameters ranged from 100 to 200 nm. To understand the effect of interfacial area on oxygen transport, a subset of NEs were prepared consisting of identical composition in which manufacturing processing pressure was varied to achieve different droplet sizes. The droplet sizes and distributions of these NEs, batches 1, 2, and 3, are shown in Figure 1A. Batch 2 and 3, prepared under identical processing pressure, exhibited highly similar size attributes, while batch 1’s lower operating pressure resulted in larger droplets. The oxygen release profiles, embodied in Figure 1B, suggest the oxygen release properties of the PFC-NEs were robust to particle size variation. Alternatively, PFC-NE composition has a notable impact on oxygen release. Oxygen release of three formulations differing in PFC concentration but identical in processing parameters are overlaid in Figure 1C. nBIONIX hypoxic pouches are sealable pouches used in combination with an oxygen scavenging agent to adjust oxygen concentration. Figure 2A demonstrates this technology was able to attain and maintain precise pO2 for at least 2 hours. Murine macrophages labeled with Hypoxia Green reagent and cultivated in the hypoxic pouches demonstrated a characteristic linear fluorescent shift with changes in pO2 (Figure 2B). This established that the fluorescent properties are attenuated at higher pO2. When macrophages cultivated at 1% pO2 were exposed to oxygenated growth medium or PFC-NE, a significant reduction of Hypoxia Green fluorescent signal was observed in the PFC-NE group (Figure 3).
Conclusion: PFC-NEs show in vitro oxygen release resistant to droplet size fluctuations and sensitive to PFC concentration. These findings are encouraging for scale-up and commercialization of such products. In macrophages, the PFC-NEs have proven effective at carrying and delivering a source of molecular oxygen in hypoxic environments. The technology utilized in this cell culture method will allow for further evaluation of these oxygen carriers.
References: 1. Riess, J. G. Oxygen carriers ("blood substitutes")--raison d'etre, chemistry, and some physiology. Chem Rev 101, 2797-2920 (2001).
2. Lambert, E. & Janjic, J. M. Quality by design approach identifies critical parameters driving oxygen delivery performance in vitro for perfluorocarbon based artificial oxygen carriers. Scientific Reports 11, 5569, doi:10.1038/s41598-021-84076-1 (2021).
Acknowledgments: The presented work was supported in part by CDMRP award number W81XWH-19-1-0828 and AFMSA cooperative agreement number FA8650-2-2-6224.
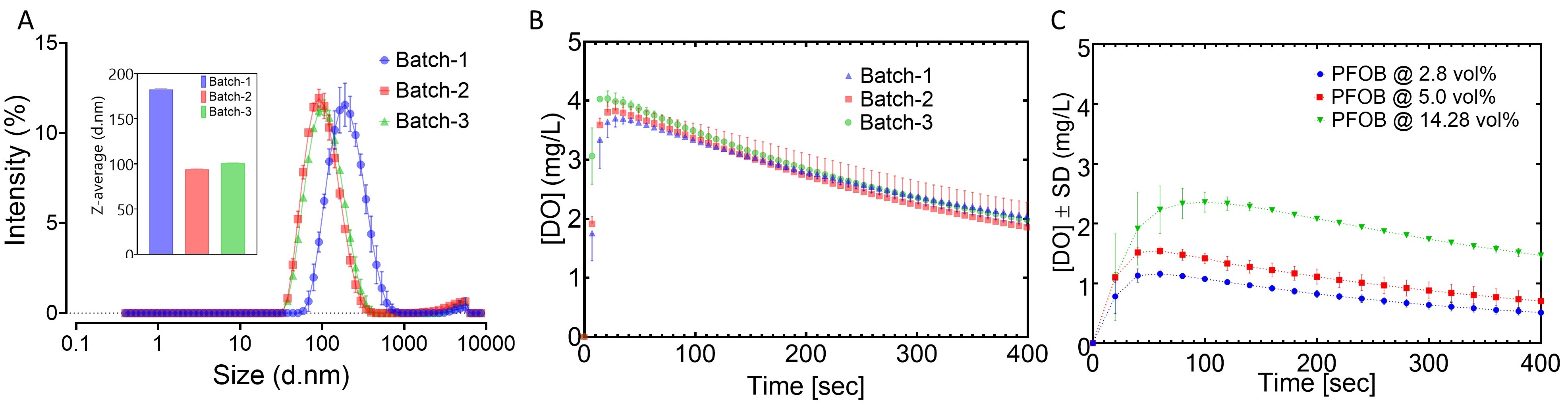
Figure 1. Demonstration of in vitro oxygen release from PFC-NEs. (A) Droplet size distributions and average size (inset) of PFC-NEs with matching composition. (B) Oxygen release profiles of PFC-NEs with matching composition. (C) Oxygen release profiles of PFC-NEs with varied composition.
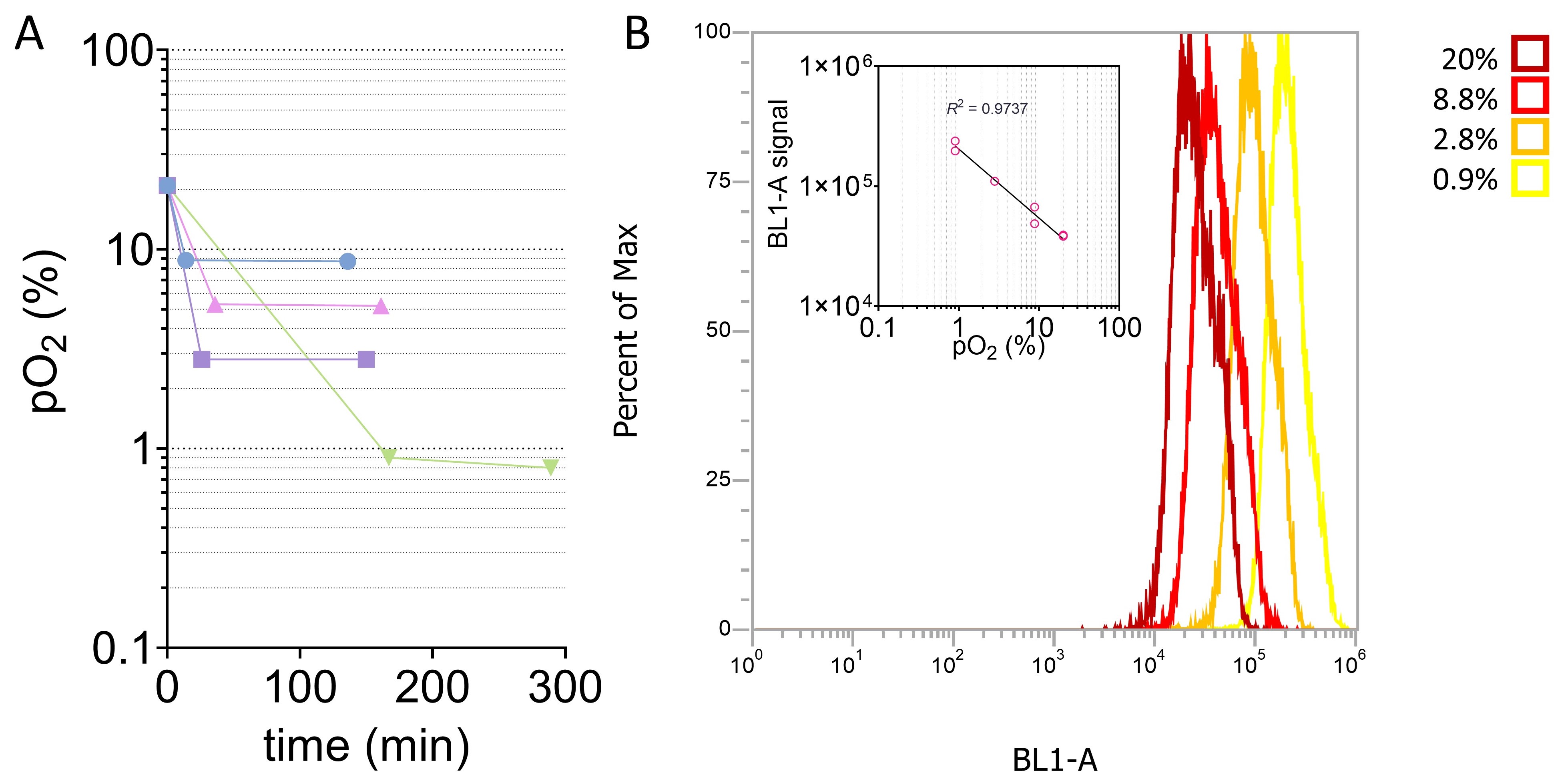
Figure 2. Evaluation of hypoxic cell culture method. (A) pO2 measured inside of hypoxic pouches set to various setpoints. (B) Fluorescence of hypoxia green-labeled macrophages decreases linearly with increasing pO2.
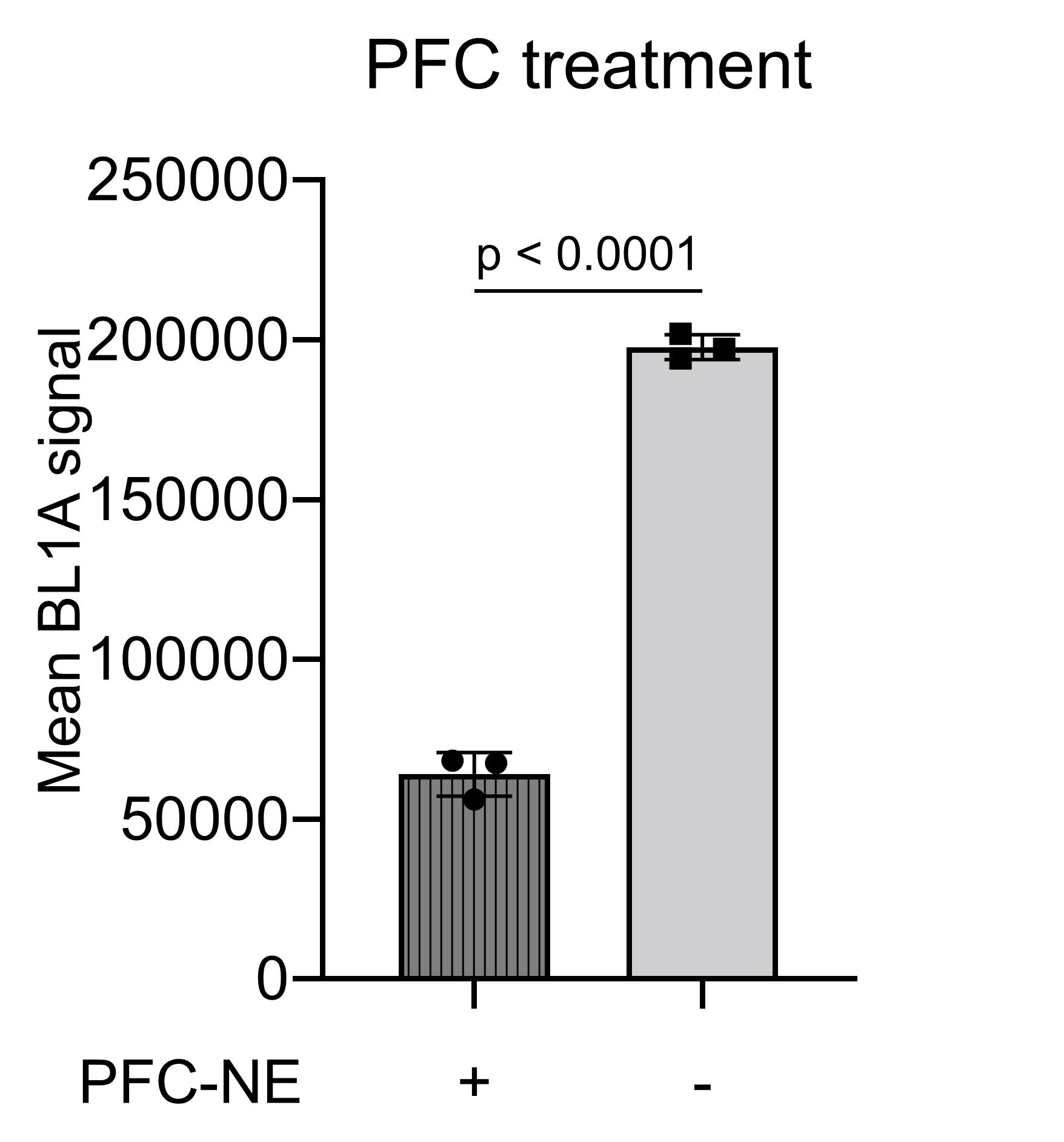
Figure 3. Flow cytometry summary of PFC-NE-treated and growth medium-treated macrophages labeled with Hypoxia Green reagent.
Formulation and Delivery - Chemical - Drug Delivery
Category: Late Breaking Poster Abstract
(M1030-12-68) Perfluorocarbon Nanoemulsions as Artificial Oxygen Carriers–Initial Proof-of-Concept to In Vitro Tissue Culture Efficacy
Monday, October 17, 2022
10:30 AM – 11:30 AM ET
- EL
Eric Lambert, BS
Duquesne University
Pittsburgh, Pennsylvania, United States - EL
Eric Lambert, BS
Duquesne University
Pittsburgh, Pennsylvania, United States
Presenting Author(s)
Main Author(s)
Purpose: Ischemia results from an array of clinical pathologies, from organ failure to chronic inflammation. In transplantation, graft endothelial cells’ structures shift in ischemic environments, leading to disruption of the endothelial wall and increased susceptibility to oxidative damage. Management of this universal hypoxic state during preservation would be a starting point to achieve better outcomes in transplant patients. Perfluorocarbons have undergone decades of development as oxygen carriers in biomedical research. They are attractive owing to the absence of infectious risk, existence of commercial manufacturing capabilities, and biological/chemical inertness1. Despite the promising pre-clinical results, there has been limited success translating perfluorocarbon-based oxygen carriers. We have taken a more rigorous approach to develop stable and pharmacologically active perfluorocarbon nanoemulsions (PFC-NEs) as oxygen carriers. Colloidal stability optimization has been previously reported2. Here, the focus is on extending our understanding of the oxygen delivery aspect of PFC-NEs.
Methods: Previously reported PFC-NEs2 served as model formulations. These were prepared in a high-energy microfluidization process and were characterized with dynamic light scattering. In vitro molecular oxygen release was evaluated by observing oxygen replenishment in a flask of deoxygenated release medium after injection of oxygenated PFC-NE into the flask. Dissolved oxygen levels were monitored using a polarographic dissolved oxygen sensor and the results were compared with a fiber optic sensor. RAW 264.7 murine macrophages were cultured in a hypoxic environment using nBIONIX hypoxic culture kits. Image-iT hypoxia green reagent, which has fluorescent properties dependent on oxygen concentration, was used as a proxy of oxygen partial pressure in the intracellular environment. Flow cytometry was utilized to evaluate macrophages labeled with hypoxia green reagent.
Results: The resulting average droplet diameters ranged from 100 to 200 nm. To understand the effect of interfacial area on oxygen transport, a subset of NEs were prepared consisting of identical composition in which manufacturing processing pressure was varied to achieve different droplet sizes. The droplet sizes and distributions of these NEs, batches 1, 2, and 3, are shown in Figure 1A. Batch 2 and 3, prepared under identical processing pressure, exhibited highly similar size attributes, while batch 1’s lower operating pressure resulted in larger droplets. The oxygen release profiles, embodied in Figure 1B, suggest the oxygen release properties of the PFC-NEs were robust to particle size variation. Alternatively, PFC-NE composition has a notable impact on oxygen release. Oxygen release of three formulations differing in PFC concentration but identical in processing parameters are overlaid in Figure 1C. nBIONIX hypoxic pouches are sealable pouches used in combination with an oxygen scavenging agent to adjust oxygen concentration. Figure 2A demonstrates this technology was able to attain and maintain precise pO2 for at least 2 hours. Murine macrophages labeled with Hypoxia Green reagent and cultivated in the hypoxic pouches demonstrated a characteristic linear fluorescent shift with changes in pO2 (Figure 2B). This established that the fluorescent properties are attenuated at higher pO2. When macrophages cultivated at 1% pO2 were exposed to oxygenated growth medium or PFC-NE, a significant reduction of Hypoxia Green fluorescent signal was observed in the PFC-NE group (Figure 3).
Conclusion: PFC-NEs show in vitro oxygen release resistant to droplet size fluctuations and sensitive to PFC concentration. These findings are encouraging for scale-up and commercialization of such products. In macrophages, the PFC-NEs have proven effective at carrying and delivering a source of molecular oxygen in hypoxic environments. The technology utilized in this cell culture method will allow for further evaluation of these oxygen carriers.
References: 1. Riess, J. G. Oxygen carriers ("blood substitutes")--raison d'etre, chemistry, and some physiology. Chem Rev 101, 2797-2920 (2001).
2. Lambert, E. & Janjic, J. M. Quality by design approach identifies critical parameters driving oxygen delivery performance in vitro for perfluorocarbon based artificial oxygen carriers. Scientific Reports 11, 5569, doi:10.1038/s41598-021-84076-1 (2021).
Acknowledgments: The presented work was supported in part by CDMRP award number W81XWH-19-1-0828 and AFMSA cooperative agreement number FA8650-2-2-6224.

Figure 1. Demonstration of in vitro oxygen release from PFC-NEs. (A) Droplet size distributions and average size (inset) of PFC-NEs with matching composition. (B) Oxygen release profiles of PFC-NEs with matching composition. (C) Oxygen release profiles of PFC-NEs with varied composition.

Figure 2. Evaluation of hypoxic cell culture method. (A) pO2 measured inside of hypoxic pouches set to various setpoints. (B) Fluorescence of hypoxia green-labeled macrophages decreases linearly with increasing pO2.

Figure 3. Flow cytometry summary of PFC-NE-treated and growth medium-treated macrophages labeled with Hypoxia Green reagent.
