Back
Purpose: Owning its effectiveness, the advance compartment and advance transit (ACAT) based computational models have become increasingly popular in the industry for predicting oral drug product performance (1). However, due to its complexity, some compromises have been made in practice, the gastric is often assigned as a single compartment. Although this assignment worked generally, it may not be sufficient to reflect the complexity of the gastric environment under certain conditions. For example, this setting was found to be less accurate in estimating stomach pH and solubilization of certain drugs under food intake leading to difficulty in predicting the food effect. To overcome the above, we explored using a kinetic pH calculation for the single compartment stomach setting. Several drugs have been tested with this new setting and results suggest this approach is effective. This approach is found to be better in estimating physicochemical properties related food effect for several basic drugs.
Methods: For drug absorption modeling, Gastoplus® (Lancaster, California, U.S) version 9.8.2 was used. For the kinetic pH modeling, The kinetic pH calculator program was implemented in Microsoft Excel® (Redmond, Washington, USA) with the following assumptions: (1) the pH time (pH (t)) profile of the stomach at a given condition (in this case after food consumption) follows first order and eventually returns to the basal level (equation 1); (2) The mass of the drug remaining in the stomach (Mass (t)) after transition to small intestine follows first-order kinetic as well (equation 2). The sum of pH (t) x Mass (t) from t=0 to T is represented as equation 3 and the Sum of Mass (t) from t=0 to T is represented as equation 4. Finally, the kinetic pH is calculated using the equation 5 and illustrated as Figure 2.
pH (t)= pH (0)*exp^(- K1*t) (Eq.1)
Mass (t) = Mass (0)*exp^(-K2*t) (Eq.2)
Sum of pH(t)*Mass(t)=pH(0)*Mass(0)+pH(1)*Mass(1)+…+pH(t-1)*Mass(t-1)+pH(t)*Mass(t) (Eq.3)
Sum of Mass(t)=Mass(0)+Mass(1)+…+Mass(t-1)+Mass(t) (Eq.4)
Kinetic pH (KpH) = Sum of pH(t)*Mass(t) / Sum of Mass(t) (Eq.5)
Where: pH (t): pH of the stomach at time t
pH (0): pH of the stomach at time 0
K1: first order rate constant for pH drop
K2: first order transit rate constant for drug transit
Mass(0): the initial dose
Mass (t): the mass of the drug in the stomach at time t
Results: The kinetic pH calculator was successfully built and the kinetic gastric pH (hereafter referred as KpH) was calculated based on separate literatures (2). In this case, reported stomach pH time profile after a high fat meal was found to have the best fit with K1 of 0.0165 min-1. With the drug transit constant (K2) of 0.01155 min-1 (3) and total simulation time of 240 minutes (4 times transit half-life)), a KpH value of 2.9 at fed state was obtained. The same approach was applied using other literature reports and found the fed KpH values was between 2.8 to 2.7. The obtained values were significantly different from the default value in Gastroplus. Therefore, the default stomach pH setting in Gastroplus may be overestimated under the fed condition (i.e. pH 4.9). This over estimation of pH with the single compartment assignment of the gastric will result in under prediction of solubilization of some basic drugs in the stomach and cause deviations. Figure 2 listed all the physiochemical properties of the compounds in our investigation (4). For all compounds, literature data (MW, diffusion coefficient, LogD/P, pKa, permeability, particle size) were entered to establish a model first, the prediction of fraction absorbed (Fa) for both Fed and Fasted were executed with the Gastroplus default settings. Following the same exercise, the KpH values of 2.9 was used to replace the default pH 4.9 for the fed conditions and predicted Fa for Fed were simulated for each compound again. A summary of the above exercise is presented as figure 3. The predicted food effect based on physicochemical properties of each compound was estimated using the Fa ratio (Fed/Fasted) and tabulated in figure 3. In general, when default gastric pH (pH 4.9 for the fed) were used for simulation, negative food effect were predicted for several compounds where the reported clinical data are all positive. On top of that, the degree of errors for several compounds are very significant. The above prediction error was significantly reduced when fed KpH was used for simulation. Most importantly, no false negative food effect was predicted.
Conclusion: Overall, using the KpH to replace the default pH performs well for predicting food effect for all test compounds. All false negative predictions were successfully corrected. Most importantly, this exercise enable us to continuously use the current ACAT model without rewriting the program or adding additional compartments for the stomach.
References: 1. Agoram B, Woltosz WS, Bolger MB. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev. 2001;50(Suppl 1):S41–S67.
2. Dressman, J. B., Berardi, R. R., Dermentzoglou, L. C., Russell, T. L., Schmaltz, S. P., Barnett, J. L., & Jarvenpaa, K. M. (1990). Upper gastrointestinal (GI) pH in young, healthy men and women. Pharmaceutical research, 7(7), 756–761
3. Sugano K. Biopharmaceutics Modeling and Simulations.2012 Wiley & Son, New Jersey, USA.
4. Chiang PC, Nagapudi K, Dolton MJ, Liu J. Exploring Multicompartment Plug Flow-Based Model Approach in Biopharmaceutics: Impact of Stomach Setting and the Estimation of the Fraction Absorbed of Orally Administered Basic Drugs. J Pharm Sci. 2020.
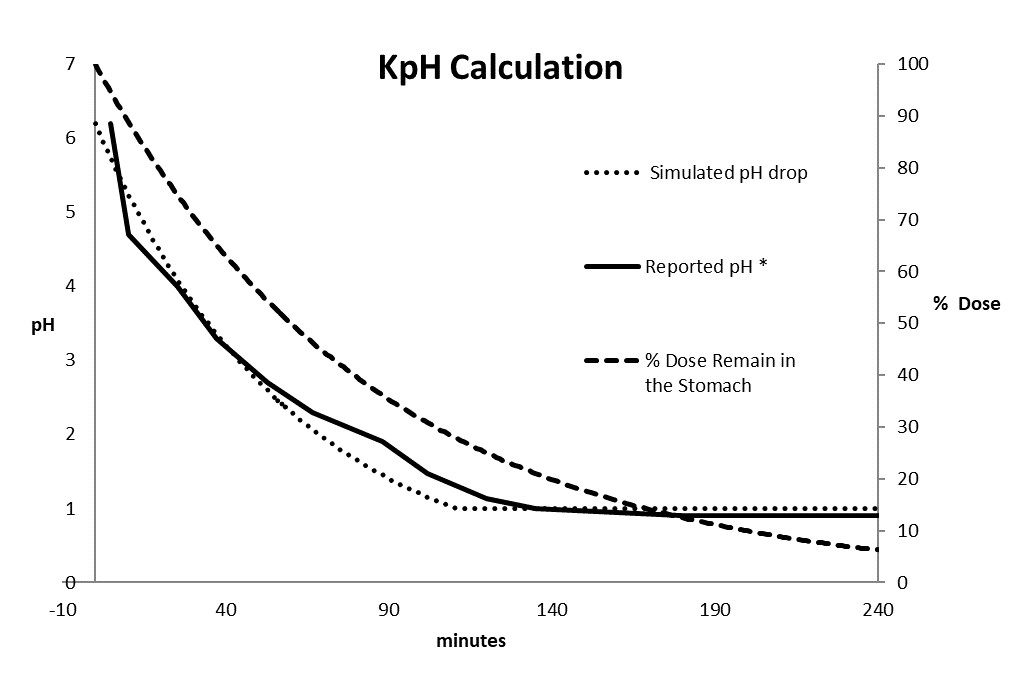
The Kinetic pH ( KpH) of Stomach (* Ref: 1)
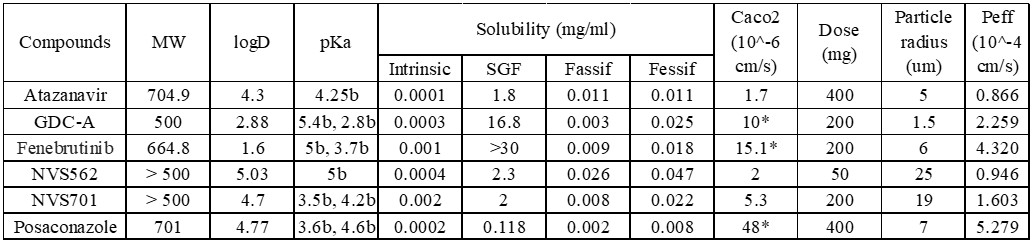
Physicochemical properties of the drugs
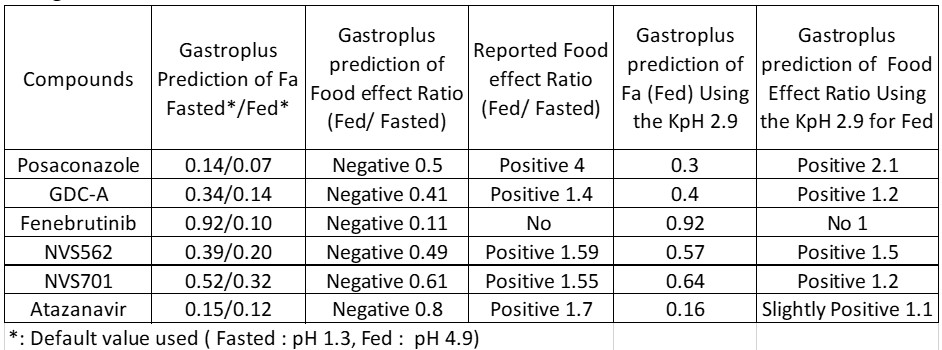
GastroPlus food effect predictions using the default gastric pH and the KpH value.
Discovery and Basic Research - Pharmaceutics
Category: Poster Abstract
(W1130-05-25) Exploring the Use of Kinetic pH Calculation to Correct the ACAT Model with a Single Stomach Compartment Setting: Impact of Stomach Setting on Food Effect Prediction for Basic Compounds
Wednesday, October 19, 2022
11:30 AM – 12:30 PM ET
- JL
Jia Liu, BS
Genentech, Inc.
South San Francisco, California, United States - PC
Po-Chang Chiang, Ph.D.
Genentech, Inc.
South San Francisco, California, United States
Presenting Author(s)
Main Author(s)
Purpose: Owning its effectiveness, the advance compartment and advance transit (ACAT) based computational models have become increasingly popular in the industry for predicting oral drug product performance (1). However, due to its complexity, some compromises have been made in practice, the gastric is often assigned as a single compartment. Although this assignment worked generally, it may not be sufficient to reflect the complexity of the gastric environment under certain conditions. For example, this setting was found to be less accurate in estimating stomach pH and solubilization of certain drugs under food intake leading to difficulty in predicting the food effect. To overcome the above, we explored using a kinetic pH calculation for the single compartment stomach setting. Several drugs have been tested with this new setting and results suggest this approach is effective. This approach is found to be better in estimating physicochemical properties related food effect for several basic drugs.
Methods: For drug absorption modeling, Gastoplus® (Lancaster, California, U.S) version 9.8.2 was used. For the kinetic pH modeling, The kinetic pH calculator program was implemented in Microsoft Excel® (Redmond, Washington, USA) with the following assumptions: (1) the pH time (pH (t)) profile of the stomach at a given condition (in this case after food consumption) follows first order and eventually returns to the basal level (equation 1); (2) The mass of the drug remaining in the stomach (Mass (t)) after transition to small intestine follows first-order kinetic as well (equation 2). The sum of pH (t) x Mass (t) from t=0 to T is represented as equation 3 and the Sum of Mass (t) from t=0 to T is represented as equation 4. Finally, the kinetic pH is calculated using the equation 5 and illustrated as Figure 2.
pH (t)= pH (0)*exp^(- K1*t) (Eq.1)
Mass (t) = Mass (0)*exp^(-K2*t) (Eq.2)
Sum of pH(t)*Mass(t)=pH(0)*Mass(0)+pH(1)*Mass(1)+…+pH(t-1)*Mass(t-1)+pH(t)*Mass(t) (Eq.3)
Sum of Mass(t)=Mass(0)+Mass(1)+…+Mass(t-1)+Mass(t) (Eq.4)
Kinetic pH (KpH) = Sum of pH(t)*Mass(t) / Sum of Mass(t) (Eq.5)
Where: pH (t): pH of the stomach at time t
pH (0): pH of the stomach at time 0
K1: first order rate constant for pH drop
K2: first order transit rate constant for drug transit
Mass(0): the initial dose
Mass (t): the mass of the drug in the stomach at time t
Results: The kinetic pH calculator was successfully built and the kinetic gastric pH (hereafter referred as KpH) was calculated based on separate literatures (2). In this case, reported stomach pH time profile after a high fat meal was found to have the best fit with K1 of 0.0165 min-1. With the drug transit constant (K2) of 0.01155 min-1 (3) and total simulation time of 240 minutes (4 times transit half-life)), a KpH value of 2.9 at fed state was obtained. The same approach was applied using other literature reports and found the fed KpH values was between 2.8 to 2.7. The obtained values were significantly different from the default value in Gastroplus. Therefore, the default stomach pH setting in Gastroplus may be overestimated under the fed condition (i.e. pH 4.9). This over estimation of pH with the single compartment assignment of the gastric will result in under prediction of solubilization of some basic drugs in the stomach and cause deviations. Figure 2 listed all the physiochemical properties of the compounds in our investigation (4). For all compounds, literature data (MW, diffusion coefficient, LogD/P, pKa, permeability, particle size) were entered to establish a model first, the prediction of fraction absorbed (Fa) for both Fed and Fasted were executed with the Gastroplus default settings. Following the same exercise, the KpH values of 2.9 was used to replace the default pH 4.9 for the fed conditions and predicted Fa for Fed were simulated for each compound again. A summary of the above exercise is presented as figure 3. The predicted food effect based on physicochemical properties of each compound was estimated using the Fa ratio (Fed/Fasted) and tabulated in figure 3. In general, when default gastric pH (pH 4.9 for the fed) were used for simulation, negative food effect were predicted for several compounds where the reported clinical data are all positive. On top of that, the degree of errors for several compounds are very significant. The above prediction error was significantly reduced when fed KpH was used for simulation. Most importantly, no false negative food effect was predicted.
Conclusion: Overall, using the KpH to replace the default pH performs well for predicting food effect for all test compounds. All false negative predictions were successfully corrected. Most importantly, this exercise enable us to continuously use the current ACAT model without rewriting the program or adding additional compartments for the stomach.
References: 1. Agoram B, Woltosz WS, Bolger MB. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev. 2001;50(Suppl 1):S41–S67.
2. Dressman, J. B., Berardi, R. R., Dermentzoglou, L. C., Russell, T. L., Schmaltz, S. P., Barnett, J. L., & Jarvenpaa, K. M. (1990). Upper gastrointestinal (GI) pH in young, healthy men and women. Pharmaceutical research, 7(7), 756–761
3. Sugano K. Biopharmaceutics Modeling and Simulations.2012 Wiley & Son, New Jersey, USA.
4. Chiang PC, Nagapudi K, Dolton MJ, Liu J. Exploring Multicompartment Plug Flow-Based Model Approach in Biopharmaceutics: Impact of Stomach Setting and the Estimation of the Fraction Absorbed of Orally Administered Basic Drugs. J Pharm Sci. 2020.

The Kinetic pH ( KpH) of Stomach (* Ref: 1)

Physicochemical properties of the drugs

GastroPlus food effect predictions using the default gastric pH and the KpH value.
