Back
Purpose: Counterfeiting of drugs is an escalating problem due to low risks for the criminal and high potential rewards. A recent report highlighted its continued growth despite the implementation of serialization attempting to secure the supply chain.1 According to a review paper by the World Health Organization (WHO), an estimated 10 to 30% of medicines are substandard or falsified in low and middle-income countries.2 These medicines cover a wide range of treatment categories including cancer medicines, contraceptives, antibiotics, vaccines and other life-saving products.3 The FDA has issued guidance to address this issue with the incorporation of physical or chemical identifiers (PCID) into solid dosage forms.4 Positive detection of the PCID would help detect counterfeit products by providing authentication and traceability to individual dosage forms. In this study, a fully formulated clear Opadry® film coating system containing a molecular taggant, as part of the SoteriaRx® on-dose authentication platform (a PCID technology), was applied to color coated acetaminophen tablets. The tagged tablets were investigated for authenticity and other performance attributes over 6 months of storage at accelerated ICH stability conditions.
Methods: Using a Labcoat I (O’Hara Technologies, Inc.) fully perforated coating pan, Opadry, Opadry II, or Opadry QX blue pigmented film coating systems, without the molecular taggant, were coated onto acetaminophen (APAP, 500 mg) to a 3% weight gain (WG) to achieve color uniformity. A top-coat clear Opadry including the molecular taggant was added to a 1% WG. After coating, the tablets were stored in induction sealed 120 mL HDPE bottles with two desiccants at 40°C/75% RH conditions over 6 months. Taggant detection was performed using a PCR portable reader based on a real-time polymerase chain reaction. Tablet color was measured analytically with a DataColor600 (DataColor, Inc.). The limit of CIELAB total color difference (DE) was defined as 2.5 for blue samples. Drug assay and drug dissolution were evaluated following USP monograph specifications.
Results: Coated and uncoated APAP samples were analyzed using real-time PCR and the resulting cycle threshold (Cq) is shown in Figure 1. A higher Cq value indicates more cycle time required to amplify the molecular taggant to reach the threshold level and therefore indicates a lower quantity of taggants. Any Cq value greater than 25 is considered non-detectable. The negative control and untagged Opadry provided a Cq >30 indicating no detection of the taggant. The molecular taggant was successfully detected through 6 months of storage at 40°C/75%RH. The tablets showed no significant change in color. The drug assay for all tablets remained within the USP specification of 90-110% of the label claim across all time points and was unaffected by the presence of a coating or by the presence of the molecular taggant. Similarly, the drug dissolution profile, as shown in Figure 2 a-e, was consistent regardless of whether the tablets were tagged or not, uncoated, or clear coated when stored at accelerated stability conditions.
Conclusion: The SoteriaRx on-dose authentication platform was used to tag acetaminophen tablets. Tagged Opadry coatings were applied as a clear top-coat over color-coated. The presence of the molecular taggant could not be visually detected, nor did it have any impact on tablet properties, such as color difference, drug assay, or drug dissolution testing at the initial time point or after storage. The positive authentication results and the minimal impact on other testing capabilities indicated that the SoteriaRx is an excellent method for an on-dose PCID authentication technology platform.
References: 1. It’s time to stop murder by counterfeit medicine. https://www.statnews.com/2019/05/07/stopping-murder-counterfeit-medicine/ April 2021
2. A Study on the Public Health and Socioeconomic Impact of Substandard and Falsified Medical Products. https://www.who.int/medicines/regulation/ssffc/publications/Layout-SEstudy-WEB.pdf?ua=1 April 2019
3. WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products. https://www.who.int/medicines/regulation/ssffc/publications/GSMS_Report_layout.pdf?ua=1
April 2019
4. Guidance for Industry - Incorporation of Physical-Chemical Identifiers into Solid Oral Dosage Form Drug Products for Anticounterfeiting. https://www.fda.gov/downloads/drugs/guidances/ucm171575.pdf April 2019
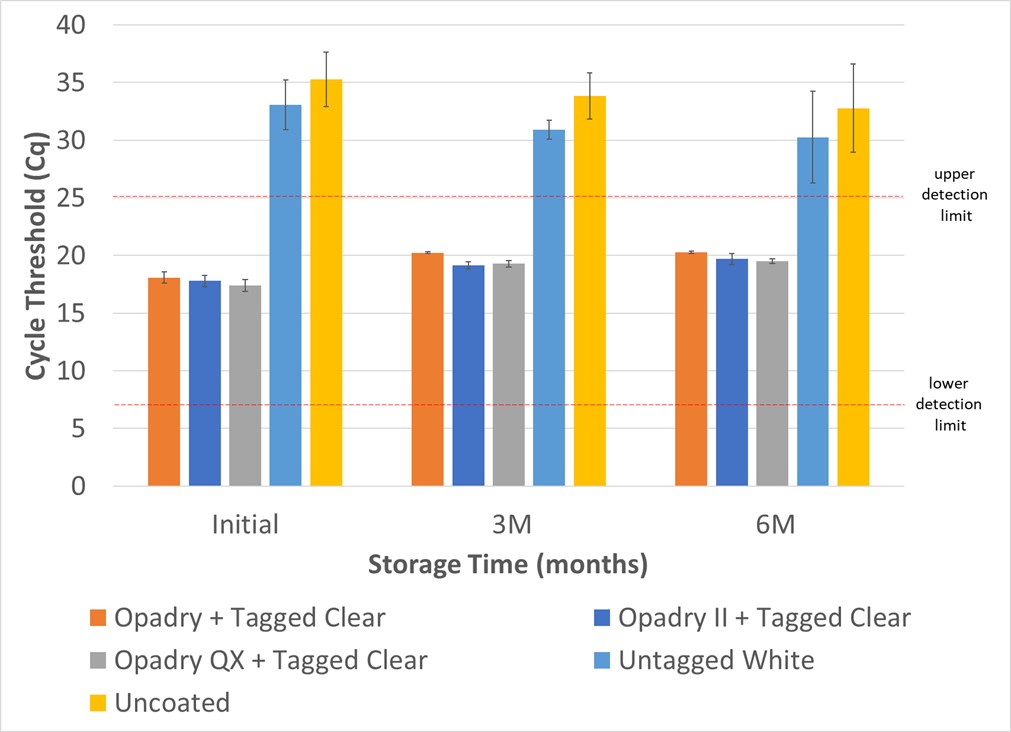
Figure 1. Cycle Threshold (Cq) for Tagged and Untagged APAP Tablets Over Stability Through 6 Months at 40°C/75%RH
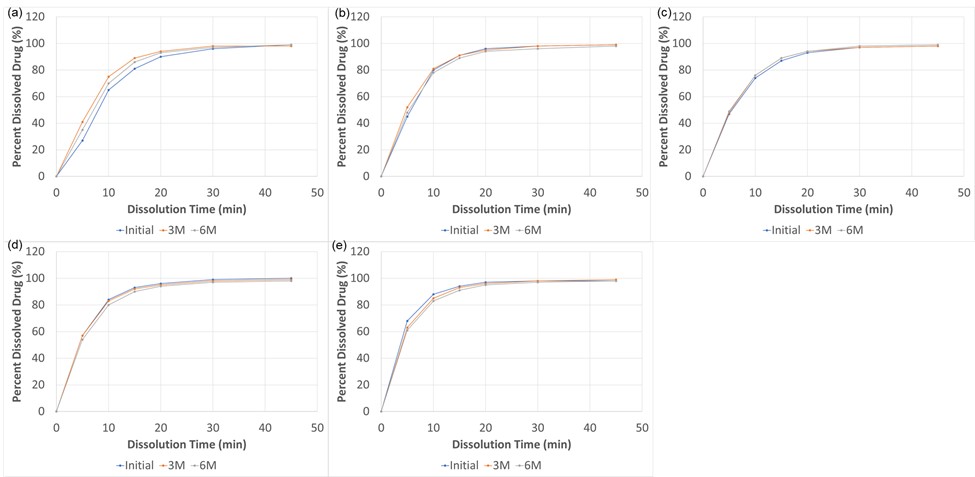
Figure 2. Drug Dissolution Profile of Tagged and Untagged Tablets Stored Through 6 Months at 40°C/75%RH (a) Blue Pigmented Opadry + with a tagged Clear Coat, (b) Blue Pigmented Opadry II + Tagged Clear Coat, (c) Blue Pigmented Opadry QX + Tagged Clear Coat, (d) White Pigmented Opadry, (e) Uncoated
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(W1030-01-05) On Dose Authentication Using Molecular Taggants Applied in a Clear Film Coat
Wednesday, October 19, 2022
10:30 AM – 11:30 AM ET
- DT
Daniel To, Ph.D.
Colorcon, Inc.
Harleysville, Pennsylvania, United States - BP
Brad Prusak
Colorcon, Inc.
Harleysville, Pennsylvania, United States
Presenting Author(s)
Main Author(s)
Purpose: Counterfeiting of drugs is an escalating problem due to low risks for the criminal and high potential rewards. A recent report highlighted its continued growth despite the implementation of serialization attempting to secure the supply chain.1 According to a review paper by the World Health Organization (WHO), an estimated 10 to 30% of medicines are substandard or falsified in low and middle-income countries.2 These medicines cover a wide range of treatment categories including cancer medicines, contraceptives, antibiotics, vaccines and other life-saving products.3 The FDA has issued guidance to address this issue with the incorporation of physical or chemical identifiers (PCID) into solid dosage forms.4 Positive detection of the PCID would help detect counterfeit products by providing authentication and traceability to individual dosage forms. In this study, a fully formulated clear Opadry® film coating system containing a molecular taggant, as part of the SoteriaRx® on-dose authentication platform (a PCID technology), was applied to color coated acetaminophen tablets. The tagged tablets were investigated for authenticity and other performance attributes over 6 months of storage at accelerated ICH stability conditions.
Methods: Using a Labcoat I (O’Hara Technologies, Inc.) fully perforated coating pan, Opadry, Opadry II, or Opadry QX blue pigmented film coating systems, without the molecular taggant, were coated onto acetaminophen (APAP, 500 mg) to a 3% weight gain (WG) to achieve color uniformity. A top-coat clear Opadry including the molecular taggant was added to a 1% WG. After coating, the tablets were stored in induction sealed 120 mL HDPE bottles with two desiccants at 40°C/75% RH conditions over 6 months. Taggant detection was performed using a PCR portable reader based on a real-time polymerase chain reaction. Tablet color was measured analytically with a DataColor600 (DataColor, Inc.). The limit of CIELAB total color difference (DE) was defined as 2.5 for blue samples. Drug assay and drug dissolution were evaluated following USP monograph specifications.
Results: Coated and uncoated APAP samples were analyzed using real-time PCR and the resulting cycle threshold (Cq) is shown in Figure 1. A higher Cq value indicates more cycle time required to amplify the molecular taggant to reach the threshold level and therefore indicates a lower quantity of taggants. Any Cq value greater than 25 is considered non-detectable. The negative control and untagged Opadry provided a Cq >30 indicating no detection of the taggant. The molecular taggant was successfully detected through 6 months of storage at 40°C/75%RH. The tablets showed no significant change in color. The drug assay for all tablets remained within the USP specification of 90-110% of the label claim across all time points and was unaffected by the presence of a coating or by the presence of the molecular taggant. Similarly, the drug dissolution profile, as shown in Figure 2 a-e, was consistent regardless of whether the tablets were tagged or not, uncoated, or clear coated when stored at accelerated stability conditions.
Conclusion: The SoteriaRx on-dose authentication platform was used to tag acetaminophen tablets. Tagged Opadry coatings were applied as a clear top-coat over color-coated. The presence of the molecular taggant could not be visually detected, nor did it have any impact on tablet properties, such as color difference, drug assay, or drug dissolution testing at the initial time point or after storage. The positive authentication results and the minimal impact on other testing capabilities indicated that the SoteriaRx is an excellent method for an on-dose PCID authentication technology platform.
References: 1. It’s time to stop murder by counterfeit medicine. https://www.statnews.com/2019/05/07/stopping-murder-counterfeit-medicine/ April 2021
2. A Study on the Public Health and Socioeconomic Impact of Substandard and Falsified Medical Products. https://www.who.int/medicines/regulation/ssffc/publications/Layout-SEstudy-WEB.pdf?ua=1 April 2019
3. WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products. https://www.who.int/medicines/regulation/ssffc/publications/GSMS_Report_layout.pdf?ua=1
April 2019
4. Guidance for Industry - Incorporation of Physical-Chemical Identifiers into Solid Oral Dosage Form Drug Products for Anticounterfeiting. https://www.fda.gov/downloads/drugs/guidances/ucm171575.pdf April 2019

Figure 1. Cycle Threshold (Cq) for Tagged and Untagged APAP Tablets Over Stability Through 6 Months at 40°C/75%RH

Figure 2. Drug Dissolution Profile of Tagged and Untagged Tablets Stored Through 6 Months at 40°C/75%RH (a) Blue Pigmented Opadry + with a tagged Clear Coat, (b) Blue Pigmented Opadry II + Tagged Clear Coat, (c) Blue Pigmented Opadry QX + Tagged Clear Coat, (d) White Pigmented Opadry, (e) Uncoated
