Back
Purpose: Pulmonary tuberculosis (TB) is a major air-borne bacterial infection caused by Mycobacterium tuberculosis (M.tb) resulting in approximately 1.3 million deaths in 2020 worldwide. Disease progression of TB is marked by the development of granulomas consisting of clusters of macrophages surrounding the mycobacteria. These granulomas are poorly vascularized and difficult to penetrate thereby causing the development of drug resistant bacterial strains. The current marketed TB vaccine (BCG) has variable efficacy (0-80%) and drug treatment involves administration of a high doses of multiple antibiotics resulting in severe systemic side effects and dose-dependent toxicity. To counter these limitations, there is an increasing need to develop antimicrobial formulations with improved specificity and localized (inhaled) delivery to primary infection site (i.e., lungs). Inhalation drug delivery offers several distinct advantages of large pulmonary surface area characterized by thin alveolar epithelium with good vasculature enabling rapid onset of drug action. Moreover, unlike oral route this can overcome first pass metabolism and enzyme activity. It provides localized drug delivery that maximizes drug concentration in lungs, and limits potential systemic adverse effects. Moreover, a dry powder formulation is more stable than liquid formulation for nebulization and allows for administering high dose of drugs to the lungs. Clofazimine is an antibiotic approved for treatment of tuberculosis and leprosy and effective against other mycobacteria. However, the main concern with pulmonary delivery of clofazimine is poor aqueous solubility of the drug. Polymer based drug delivery system can be an effective strategy to deliver clofazimine. Poly (lactic acid-co-glycolic acid) (PLGA) microparticles offer improved solubility, sustained drug release and allow passive and active targeting to alveolar macrophages. Therefore, we propose to develop dry powder poly (lactic acid-co-glycolic acid) (PLGA) microparticles of clofazimine in this study.
Methods: Clofazimine microparticles (CFZ MPs) were prepared by a single emulsion solvent evaporation technique. Briefly, PLGA and CFZ (20:1) were dissolved in dichloromethane to prepare the primary organic phase. The organic phase was then added to 1% w/v polyvinyl alcohol solution under homogenization for 12 min at 10000 rpm to form a uniform primary emulsion. The obtained emulsion is subjected to stirring at 500 rpm for 3 hours to remove the solvent and harden the MPs. Further, the colloidal micro suspension was centrifuged to remove unentrapped drug. Finally, the microparticulate pellet was redispersed in deionized water and spray dried with inert carrier (L-Leucine) using Buchi Mini Spray Dryer B-290 (Buchi Laboratory Equipment, Flawil, Switzerland) to obtain dry powder formulation of clofazimine spray-dried microparticles (CFZSDMP). The total solid content was 2.5%w/w. The characterization was performed using Differential Scanning Calorimetry, Powder X-Ray diffraction studies and Thermogravimetric Analysis. Further, the formulation was analyzed for in vitro release and in vitro aerosolization performance (Next Generation Impactor). In addition, bacterial minimum inhibitory concentration (MIC) studies were conducted on H35Ra avirulent strain to evaluate the effectiveness of formulation.
Results: Clofazimine microparticles were prepared with a size of 1-1.85 µm, 0.62±0.02 PDI and zeta-potential of -31.42±5.30 mV. The entrapment efficiency and drug loading of clofazimine in microparticles was 70.89±4.33 %w/w and 35.43±2.29 µg/mg. The spray-dried formulation resulted in dry powder with a yield of 70.04±3.67 %w/w. Solid state characterization of CFZ with PXRD showed that it has characteristic crystalline peaks at 19˚, 20˚ and 21˚. However, in CFZSDMP the crystalline peaks of CFZ were absent demonstrating encapsulation of drug in polymer (figure 1a). DSC thermographs represented in figure 1b exhibited sharp melting endotherm of clofazimine at 220 °C. However, the DSC thermograph of blank MP spray dried formulation with L-leucine indicated a melting endothermic peak at 218 °C. This melting endothermic peak was corresponding to degradation of L-Leucine. Likewise, the DSC curve of CFZSDMP also depicted the L-Leucine degradation melting endotherm. Additional TGA analysis (figure 1c) confirmed L-Leucine undergoing degradation at 218 °C marked by steep decrease in % weight of the sample. The dry powder formulation showed an initial burst release of 44.34% in 12h and complete release within 72h (figure 2a). Samples showed good aerosolization properties during the in vitro aerodynamic measurements with fine particle fraction (FPF) of about 86.45±0.21% and mass median aerodynamic diameter (MMAD) of 1.56±0.11 µm (figure 2b and c). Aerodynamic properties show effective deposition in the deeper airways. Additionally, the formulation also exhibited high stability and retained CFZ in amorphous form even after 4 weeks. The MIC of the free drug and CFZSDMP on H37Ra was 0.5 µg/mL and 0.0625 µg/mL, which showed that formulation was more effective in inhibiting bacterial growth compared to free drug.
Conclusion: Inhaled dry powder formulation of clofazimine microparticles were developed via spray drying with effective anti-mycobacterial TB activity. The spray dried CFZ formulation demonstrated lower MIC, good aerosolization properties with high stability at 4˚ C and 25˚ C even after 4 weeks there by presenting an immense potential for the treatment of tuberculosis.
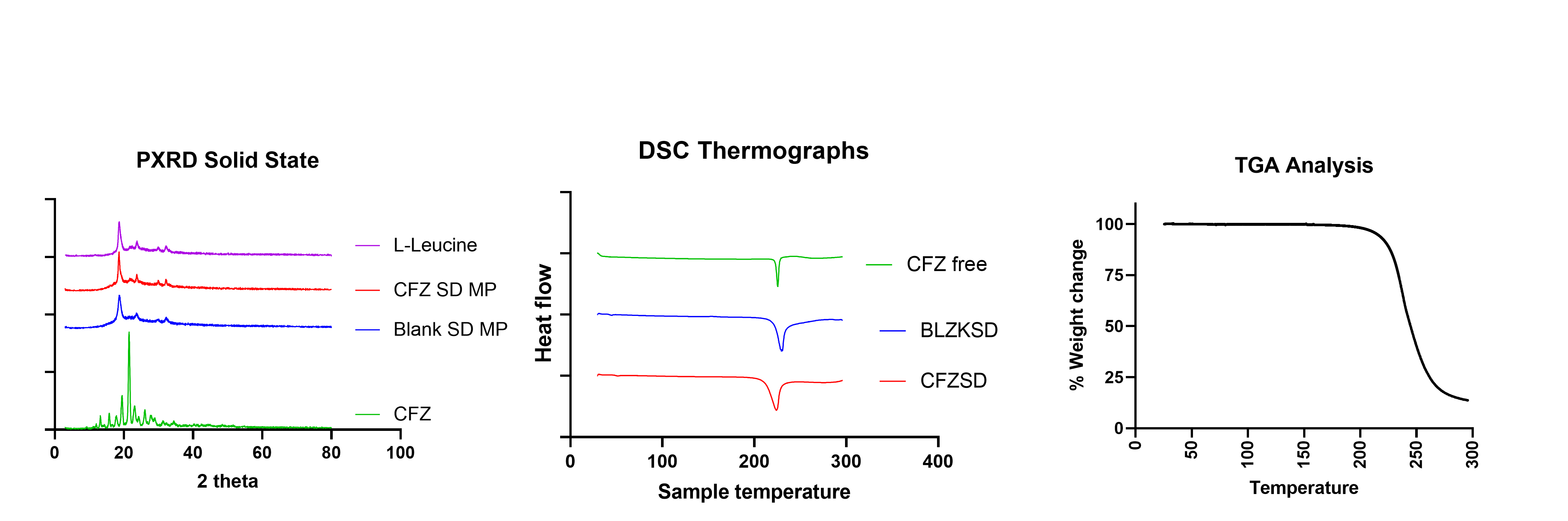
Figure 1 a) PXRD diffractogram of CFZ SD microparticle indicating complete encapsulation of drug into the MP b) DSC thermograph of CFZ SD microparticle shows clofazimine characteristic melting peak at 218 ˚C. b) TGA of CFZSD microparticle shows leucine degradation occurring from 220 ˚C
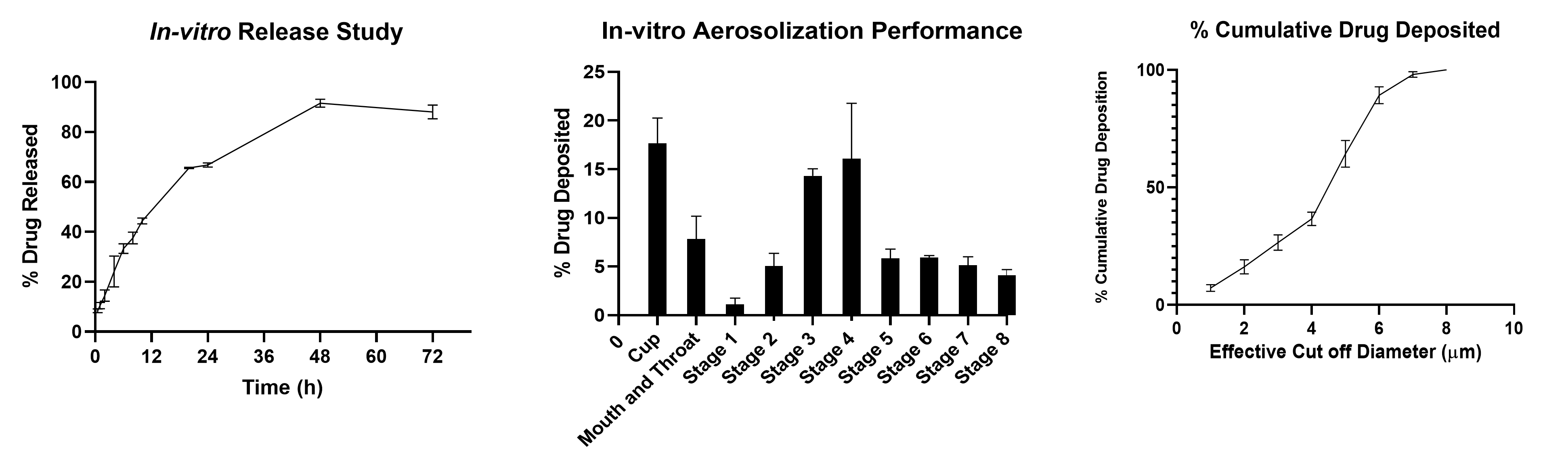
Figure 2 a) In-vitro release of CFZ from CFZ SD microparticle indicating burst release of 44.34 % about in 12 h and complete release within 72 h. b) In vitro aerosol deposition profile of CFZ-MP represented as percent drug deposited on each stage of the Next Generation Impactor (NGI). c) % cumulative drug deposition profile of CFZ-MP represented Data represented as Mean ± SD, n = 3.
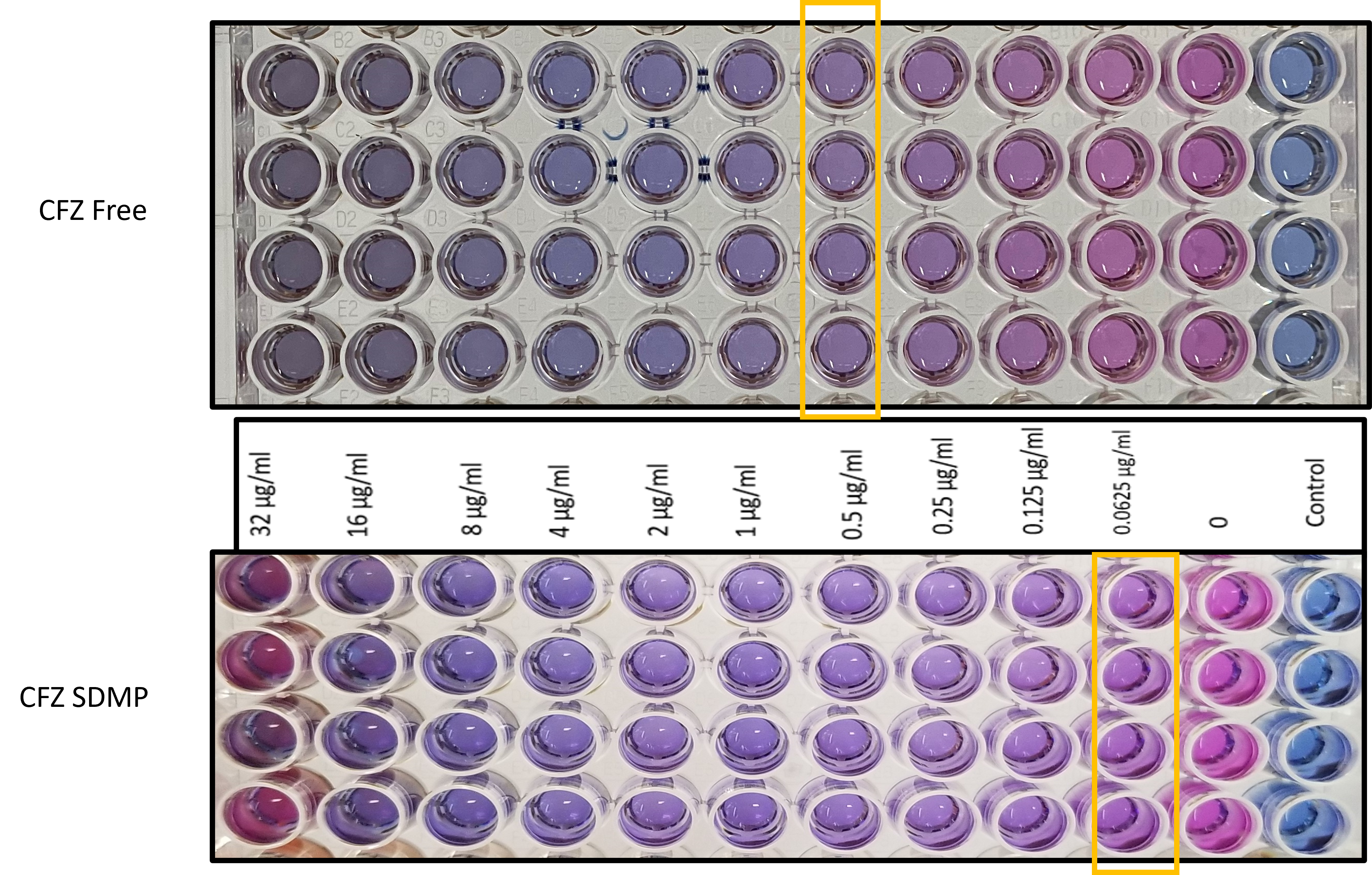
Figure 3: Minimum inhibitory concentration (MIC) determination using resazurin-based microtiter plate (REMA) assay
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(W1030-04-19) Inhalable Dry Powder Formulation of PLGA Microparticles Loaded with Clofazimine for Treatment of Tuberculosis
Wednesday, October 19, 2022
10:30 AM – 11:30 AM ET
- DB
Druva Sarika Barji, MS
St. John's University
Queens, New York, United States - DB
Druva Sarika Barji, MS
St. John's University
Queens, New York, United States
Presenting Author(s)
Main Author(s)
Purpose: Pulmonary tuberculosis (TB) is a major air-borne bacterial infection caused by Mycobacterium tuberculosis (M.tb) resulting in approximately 1.3 million deaths in 2020 worldwide. Disease progression of TB is marked by the development of granulomas consisting of clusters of macrophages surrounding the mycobacteria. These granulomas are poorly vascularized and difficult to penetrate thereby causing the development of drug resistant bacterial strains. The current marketed TB vaccine (BCG) has variable efficacy (0-80%) and drug treatment involves administration of a high doses of multiple antibiotics resulting in severe systemic side effects and dose-dependent toxicity. To counter these limitations, there is an increasing need to develop antimicrobial formulations with improved specificity and localized (inhaled) delivery to primary infection site (i.e., lungs). Inhalation drug delivery offers several distinct advantages of large pulmonary surface area characterized by thin alveolar epithelium with good vasculature enabling rapid onset of drug action. Moreover, unlike oral route this can overcome first pass metabolism and enzyme activity. It provides localized drug delivery that maximizes drug concentration in lungs, and limits potential systemic adverse effects. Moreover, a dry powder formulation is more stable than liquid formulation for nebulization and allows for administering high dose of drugs to the lungs. Clofazimine is an antibiotic approved for treatment of tuberculosis and leprosy and effective against other mycobacteria. However, the main concern with pulmonary delivery of clofazimine is poor aqueous solubility of the drug. Polymer based drug delivery system can be an effective strategy to deliver clofazimine. Poly (lactic acid-co-glycolic acid) (PLGA) microparticles offer improved solubility, sustained drug release and allow passive and active targeting to alveolar macrophages. Therefore, we propose to develop dry powder poly (lactic acid-co-glycolic acid) (PLGA) microparticles of clofazimine in this study.
Methods: Clofazimine microparticles (CFZ MPs) were prepared by a single emulsion solvent evaporation technique. Briefly, PLGA and CFZ (20:1) were dissolved in dichloromethane to prepare the primary organic phase. The organic phase was then added to 1% w/v polyvinyl alcohol solution under homogenization for 12 min at 10000 rpm to form a uniform primary emulsion. The obtained emulsion is subjected to stirring at 500 rpm for 3 hours to remove the solvent and harden the MPs. Further, the colloidal micro suspension was centrifuged to remove unentrapped drug. Finally, the microparticulate pellet was redispersed in deionized water and spray dried with inert carrier (L-Leucine) using Buchi Mini Spray Dryer B-290 (Buchi Laboratory Equipment, Flawil, Switzerland) to obtain dry powder formulation of clofazimine spray-dried microparticles (CFZSDMP). The total solid content was 2.5%w/w. The characterization was performed using Differential Scanning Calorimetry, Powder X-Ray diffraction studies and Thermogravimetric Analysis. Further, the formulation was analyzed for in vitro release and in vitro aerosolization performance (Next Generation Impactor). In addition, bacterial minimum inhibitory concentration (MIC) studies were conducted on H35Ra avirulent strain to evaluate the effectiveness of formulation.
Results: Clofazimine microparticles were prepared with a size of 1-1.85 µm, 0.62±0.02 PDI and zeta-potential of -31.42±5.30 mV. The entrapment efficiency and drug loading of clofazimine in microparticles was 70.89±4.33 %w/w and 35.43±2.29 µg/mg. The spray-dried formulation resulted in dry powder with a yield of 70.04±3.67 %w/w. Solid state characterization of CFZ with PXRD showed that it has characteristic crystalline peaks at 19˚, 20˚ and 21˚. However, in CFZSDMP the crystalline peaks of CFZ were absent demonstrating encapsulation of drug in polymer (figure 1a). DSC thermographs represented in figure 1b exhibited sharp melting endotherm of clofazimine at 220 °C. However, the DSC thermograph of blank MP spray dried formulation with L-leucine indicated a melting endothermic peak at 218 °C. This melting endothermic peak was corresponding to degradation of L-Leucine. Likewise, the DSC curve of CFZSDMP also depicted the L-Leucine degradation melting endotherm. Additional TGA analysis (figure 1c) confirmed L-Leucine undergoing degradation at 218 °C marked by steep decrease in % weight of the sample. The dry powder formulation showed an initial burst release of 44.34% in 12h and complete release within 72h (figure 2a). Samples showed good aerosolization properties during the in vitro aerodynamic measurements with fine particle fraction (FPF) of about 86.45±0.21% and mass median aerodynamic diameter (MMAD) of 1.56±0.11 µm (figure 2b and c). Aerodynamic properties show effective deposition in the deeper airways. Additionally, the formulation also exhibited high stability and retained CFZ in amorphous form even after 4 weeks. The MIC of the free drug and CFZSDMP on H37Ra was 0.5 µg/mL and 0.0625 µg/mL, which showed that formulation was more effective in inhibiting bacterial growth compared to free drug.
Conclusion: Inhaled dry powder formulation of clofazimine microparticles were developed via spray drying with effective anti-mycobacterial TB activity. The spray dried CFZ formulation demonstrated lower MIC, good aerosolization properties with high stability at 4˚ C and 25˚ C even after 4 weeks there by presenting an immense potential for the treatment of tuberculosis.

Figure 1 a) PXRD diffractogram of CFZ SD microparticle indicating complete encapsulation of drug into the MP b) DSC thermograph of CFZ SD microparticle shows clofazimine characteristic melting peak at 218 ˚C. b) TGA of CFZSD microparticle shows leucine degradation occurring from 220 ˚C

Figure 2 a) In-vitro release of CFZ from CFZ SD microparticle indicating burst release of 44.34 % about in 12 h and complete release within 72 h. b) In vitro aerosol deposition profile of CFZ-MP represented as percent drug deposited on each stage of the Next Generation Impactor (NGI). c) % cumulative drug deposition profile of CFZ-MP represented Data represented as Mean ± SD, n = 3.

Figure 3: Minimum inhibitory concentration (MIC) determination using resazurin-based microtiter plate (REMA) assay
