Back
Purpose: In 2022, there will be an estimated 1.9 million new cancer cases diagnosed and 609,360 cancer deaths only in the United States (according to cancer.org). Tumor specific delivery of chemotherapeutics with less systemic side effects, is an ongoing challenge of chemotherapy. In this context, we proposed a cisplatin (CisPt)-loaded sodium hyaluronate (NaHA) gel for intratumoral therapy. Therefore, this study addresses the idea to deliver CisPt/NaHA complex locally in different cancer, where CD44 receptor is over expressed. Hyaluronic acid is the primary CD44 binding molecule and in many studies has proved a significant ally in developing drug delivery system that demonstrate preferential tumor accumulation and increased cell uptake. To this aim, we investigated in vitro the anticancer efficacy of cisplatin/hyaluronan complex vs. CisPt alone and the possible enhancement by valproic acid (VA) using 2D and 3D cell models of various cancer cell lines overexpressing CD44. Also, effect of drug exposure time on cell viability of various cancer cell lines was evaluated.
Methods: The anti-proliferative effects of cisplatin/hyaluronan complex and cisplatin with valproic acid was determined in human lung epithelial cell (A459), human pancreatic cancer cell line (MiaPaCa-2 and BxPC3) and human melanoma cell line (A375 and vemurafenib-resistant melanoma cells (AV)) by MTT cytotoxicity assay after 48h. Effect of drug exposure time on cell viability was assessed. The CisPt/NaHA complex was formulated in NaCl saline using hyaluronan at low (LMW) and high (HMW) molecular weight, namely 10 kDa and 1.330 kDa. Complex formation was assessed by HPLC. Growth and morphology of CisPt/NaHA complex and complex with valproic acid was evaluated also in 3D multicellular melanoma tumor spheroids.
Results: Cisplatin, CisPt/NaHA complex (high molecular weight of NaHA) and CisPt with valproic acid exhibited dose-dependent inhibition in viability of all cancer cells tested (IC50 values reported in Table 1). There was a significant difference in cisplatin-NaHA induced cell killing in cancer cell line. In this in vitro model, the complex with HMW NaHA was as effective as CisPt alone at 48h. The same result was observed by shortening treatment to 2 or 4 hours. In the concentrations used, 76% of cisplatin was complexed with NaHA, thus only 24% of CisPt was immediately available. It could be that the high molecular weight of NAHA used hindered CisPt uptake by cells. Therefore, expanding on the effect of HA molecular weight, the complex was re-formulated using LMW HA and was applied for 4 and 24 hours only at 2 and 10µM CisPt concentrations. The results are reported in Figure 1. It is important to mind that in case of the complex, coordinated CisPt may not be immediately available for cell uptake. In A459 cell line a greater efficacy of the complex was observed, not seen before with HMW NaHA. Similarly, in A375 cell line we observed a significant cytotoxic effect with CisPt/NaHA complex in comparison with cisplatin alone, both after 4 and 24 hours of treatment exposure. Moreover, this cell line was sensitive to the addition of valproic acid. Based on the above, we selected the melanoma cell line (A375 and AV, the resistant one) for further study with 3D multicellular melanoma spheroids. In this we observed significant reduction in the area of spheroids for the CisPt/NaHA complex and for the CisPt/NaHA complex plus valproic acid groups in comparison with control. Spheroids surface in CisPt/NaHA complex group exhibited rough morphology indicating its cytotoxic effect while control spheroids were continuously growing and exhibited a smooth and uniform surface. This different morphology and significant reduction in area of spheroids associated with the CisPt/NaHA complex and CisPt/NaHA plus valproic acid treatment groups indicate their strong antitumor efficacy as compared to cisplatin alone in vitro (Figure 2).
Conclusion: Overall, this study confirms the efficacy of CisPt/NaHA complex in all the cell lines tested and the possibility to make a combination with valproic acid in order to increase efficacy. These preliminary results open to the next study of CisPt/NaHA complex, delivered in gel form to a murine model of melanoma. The gel form allows to inject the complex, possibly also with valproic acid, directly in the mass tumor.
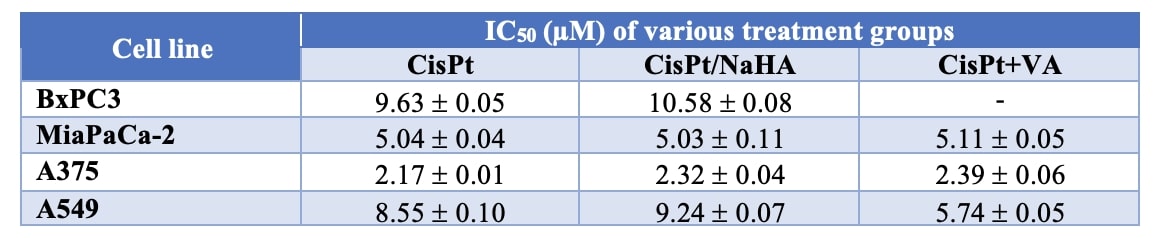
Table 1. IC50 (µM) of CisPt, CisPt/NaHA and CisPt+VA in BxPC3, MiaPaCa-2, A375, A549.
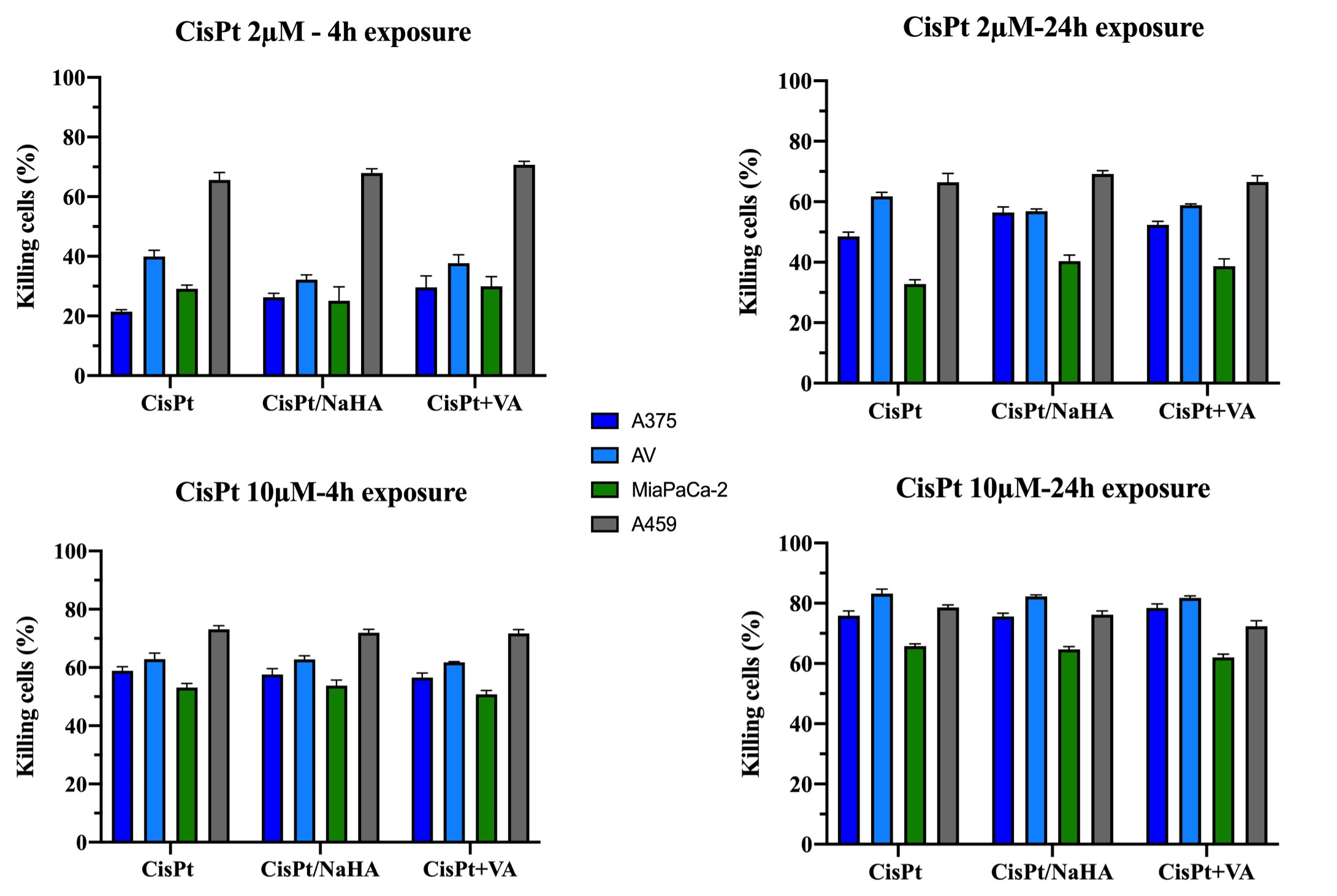
Figure 1. In vitro cytotoxicity of cisplatin (CisPt), cisplatin/hyaluronan (CisPt/NaHA) complex and cisplatin plus valproic acid (CisPt+VA) in the following cell lines: A375, AV, MiaPaCa-2 and A549.
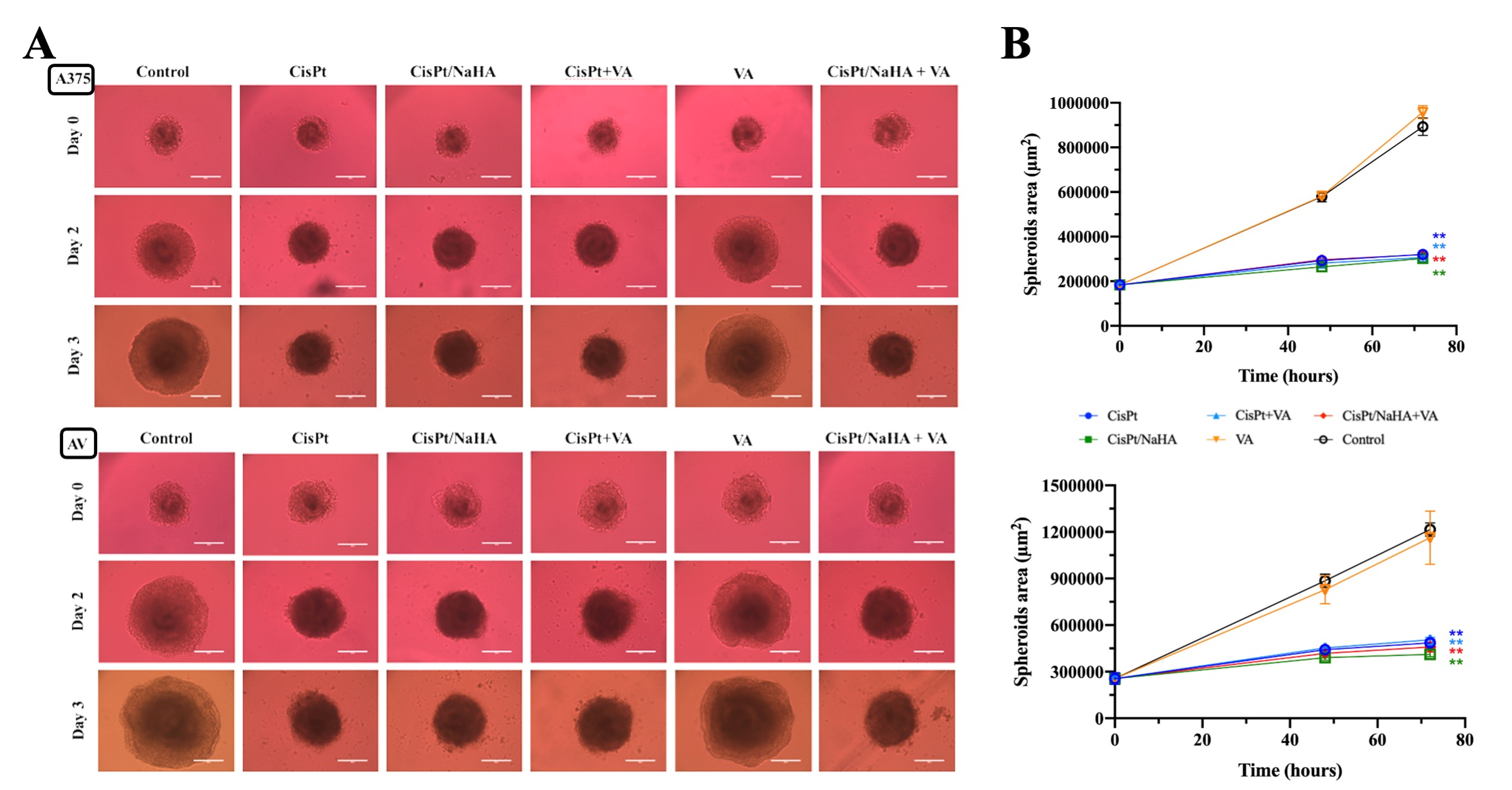
Figure 2. (A) Representative images of spheroids treated with control, CisPt, CisPt/NaHA, CisPt+VA, VA and CisPt/NaHA+VA on days 0-3 of treatment in A375 (upper) and in resistant melanoma (bottom). (B) Comparison of the area of spheroids (A375 upper; resistant melanoma bottom) treated with different treatment groups during the 72 hours of treatment. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001)
Formulation and Delivery - Biomolecular - Drug Delivery
Category: Poster Abstract
(W0930-04-19) Cisplatin/Hyaluronan Complex: A Novel Loco-Regional Treatment in Tumors Over-Expressing CD44 Receptor
Wednesday, October 19, 2022
9:30 AM – 10:30 AM ET
- SB
Sabrina Banella
University of Ferrara
Ferrara, Emilia-Romagna, Italy - SB
Sabrina Banella
University of Ferrara
Ferrara, Emilia-Romagna, Italy
Presenting Author(s)
Main Author(s)
Purpose: In 2022, there will be an estimated 1.9 million new cancer cases diagnosed and 609,360 cancer deaths only in the United States (according to cancer.org). Tumor specific delivery of chemotherapeutics with less systemic side effects, is an ongoing challenge of chemotherapy. In this context, we proposed a cisplatin (CisPt)-loaded sodium hyaluronate (NaHA) gel for intratumoral therapy. Therefore, this study addresses the idea to deliver CisPt/NaHA complex locally in different cancer, where CD44 receptor is over expressed. Hyaluronic acid is the primary CD44 binding molecule and in many studies has proved a significant ally in developing drug delivery system that demonstrate preferential tumor accumulation and increased cell uptake. To this aim, we investigated in vitro the anticancer efficacy of cisplatin/hyaluronan complex vs. CisPt alone and the possible enhancement by valproic acid (VA) using 2D and 3D cell models of various cancer cell lines overexpressing CD44. Also, effect of drug exposure time on cell viability of various cancer cell lines was evaluated.
Methods: The anti-proliferative effects of cisplatin/hyaluronan complex and cisplatin with valproic acid was determined in human lung epithelial cell (A459), human pancreatic cancer cell line (MiaPaCa-2 and BxPC3) and human melanoma cell line (A375 and vemurafenib-resistant melanoma cells (AV)) by MTT cytotoxicity assay after 48h. Effect of drug exposure time on cell viability was assessed. The CisPt/NaHA complex was formulated in NaCl saline using hyaluronan at low (LMW) and high (HMW) molecular weight, namely 10 kDa and 1.330 kDa. Complex formation was assessed by HPLC. Growth and morphology of CisPt/NaHA complex and complex with valproic acid was evaluated also in 3D multicellular melanoma tumor spheroids.
Results: Cisplatin, CisPt/NaHA complex (high molecular weight of NaHA) and CisPt with valproic acid exhibited dose-dependent inhibition in viability of all cancer cells tested (IC50 values reported in Table 1). There was a significant difference in cisplatin-NaHA induced cell killing in cancer cell line. In this in vitro model, the complex with HMW NaHA was as effective as CisPt alone at 48h. The same result was observed by shortening treatment to 2 or 4 hours. In the concentrations used, 76% of cisplatin was complexed with NaHA, thus only 24% of CisPt was immediately available. It could be that the high molecular weight of NAHA used hindered CisPt uptake by cells. Therefore, expanding on the effect of HA molecular weight, the complex was re-formulated using LMW HA and was applied for 4 and 24 hours only at 2 and 10µM CisPt concentrations. The results are reported in Figure 1. It is important to mind that in case of the complex, coordinated CisPt may not be immediately available for cell uptake. In A459 cell line a greater efficacy of the complex was observed, not seen before with HMW NaHA. Similarly, in A375 cell line we observed a significant cytotoxic effect with CisPt/NaHA complex in comparison with cisplatin alone, both after 4 and 24 hours of treatment exposure. Moreover, this cell line was sensitive to the addition of valproic acid. Based on the above, we selected the melanoma cell line (A375 and AV, the resistant one) for further study with 3D multicellular melanoma spheroids. In this we observed significant reduction in the area of spheroids for the CisPt/NaHA complex and for the CisPt/NaHA complex plus valproic acid groups in comparison with control. Spheroids surface in CisPt/NaHA complex group exhibited rough morphology indicating its cytotoxic effect while control spheroids were continuously growing and exhibited a smooth and uniform surface. This different morphology and significant reduction in area of spheroids associated with the CisPt/NaHA complex and CisPt/NaHA plus valproic acid treatment groups indicate their strong antitumor efficacy as compared to cisplatin alone in vitro (Figure 2).
Conclusion: Overall, this study confirms the efficacy of CisPt/NaHA complex in all the cell lines tested and the possibility to make a combination with valproic acid in order to increase efficacy. These preliminary results open to the next study of CisPt/NaHA complex, delivered in gel form to a murine model of melanoma. The gel form allows to inject the complex, possibly also with valproic acid, directly in the mass tumor.

Table 1. IC50 (µM) of CisPt, CisPt/NaHA and CisPt+VA in BxPC3, MiaPaCa-2, A375, A549.

Figure 1. In vitro cytotoxicity of cisplatin (CisPt), cisplatin/hyaluronan (CisPt/NaHA) complex and cisplatin plus valproic acid (CisPt+VA) in the following cell lines: A375, AV, MiaPaCa-2 and A549.

Figure 2. (A) Representative images of spheroids treated with control, CisPt, CisPt/NaHA, CisPt+VA, VA and CisPt/NaHA+VA on days 0-3 of treatment in A375 (upper) and in resistant melanoma (bottom). (B) Comparison of the area of spheroids (A375 upper; resistant melanoma bottom) treated with different treatment groups during the 72 hours of treatment. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001)
