Back
Purpose: PDAC is an aggressive malignant neoplasm which continues to carry very poor prognosis despite advances in therapies over the past decade. The PDAC stroma-tumor microenvironment contributes to resistance mechanisms for optimum drug delivery and antitumor activity. Calcipotriol (Cal) is a stroma modulator, that is hypothesized to enable better drug delivery of paclitaxel (PTX) into the tumor tissues and enhance its cancer cell cytolytic activity. We recently developed and validated a UPLC-MS/MS assay for simultaneous quantifications of Cal and PTX at LLOQ of 0.5 ng/ml and developed a nano-polymeric micelle drug delivery system, loaded with Cal and PTX (M-Cal/PTX), for treatment of PDAC. Biodistribution studies using the micellar system confirmed Cal/PTX accumulation in orthotopic mouse PDAC models. The pharmacodynamics (efficacy) and safety assessments of M-Cal/PTX in the mouse PDAC model are presented herein.
Methods: To perform short-term efficacy, and safety studies, we used orthotopic Kras* mouse model of PDAC. The primary endpoints were to assess: 1) tumor response as measured by T2-MRI; and 2) the change in circulating stroma markers with M-Cal/PTX treatment. Mice received IV doses of M-Cal/PTX (0.5 mg/kg Cal and 5 mg/kg PTX); the dosing scheme used is summarized in Figure 1. Mono formulation of Cal (M-Cal) (N=3), a mixture of mono-formulated Cal and mono-formulated PTX (M-Cal + M-PTX) (N=4), a combination of M-Cal and Abraxane (M-Cal + ABX) (N=3) and a cremophor formulation of Cal and PTX (Crem-Cal + PTX) (N=6) were included as controls. The tumor size was measured by means of T2-MRI and the corresponding volumetric quantifications were obtained. The effects of treatment on the circulating levels of TIMP1, TSP2 and MMP7 were evaluated by ELISA. A survival study was conducted to estimate the treatment benefit in terms of overall survival.
Results: In the M-Cal/PTX group, there was a 75% response and one animal had significant tumor progression. In contrast, 75% of mice in the sham group showed tumor progression (Figure 2). Similarly, the M-Cal/PTX treatment benefit was demonstrated by assessing tumor volume (Figure 3A). Two animals in the M-Cal/PTX group showed a moderate increase in their tumor volumes from day 7 while one had tumor shrinkage at days 7 and 14. None of the animals in the sham group has tumor shrinkage. The effects of M-Cal/PTX on circulating plasma markers of stroma activity were measured. For TIMP1 a delayed response was observed (Figure 3B). The mean TIMP1 levels continued to decrease after the 4th dose (day 7) while the levels remained very high in the sham group. The effect of M-Cal/PTX on circulating TSP2 levels was rapid (Figure 3C). Reduction of TSP2 to baseline levels was achieved after the second dose and the levels remained low through the 10th dose. M-Cal/PTX did not impact the circulating levels of MMP7 after 10 doses. Treatment with M-Cal/PTX caused significant decrease on alpha-smooth muscle actin (𝛼-SMA), another reliable marker for stromal activity, compared with the sham group. We looked at the Ki-67 (a proliferation marker) and matrix protein collagen, and both were significantly inhibited in the treatment group compared to the sham group. For survival analysis, animals that were administered M-Cal/PTX lived the longest (median of 54 days) compared to of 24 days for those received sham (Figure 3D). We finally examined the effects of treatment on body weight and other markers of organ health (albumin, liver enzymes, blood urea nitrogen, globulin, and total protein). No significant change in weight with M-Cal/PTX compared to the sham group, and no notable abnormalities in the laboratory markers in the treatment group.
Conclusion: Our data shows that M-Cal/PTX is a potentially effective combination regimen for treatment of PDAC. Compared to sham and other treatment interventions, M-Cal/PTX was associated with reduction of stroma-related markers, better antitumor activity, and improvement in median survival of tumor-bearing mice. Furthermore, no changes in mouse weight in the M-Cal/PTX group or notable laboratory abnormalities suggesting a tolerable safety profile for this combination therapy at the tested dose. Additional preclinical studies are in progress to further explore and develop this optimal regimen for PDAC treatment.
.jpg)
Figure 1. Dosing scheme for the evaluation of short-term benefit of M-Cal/PTX in a mouse model of PDAC
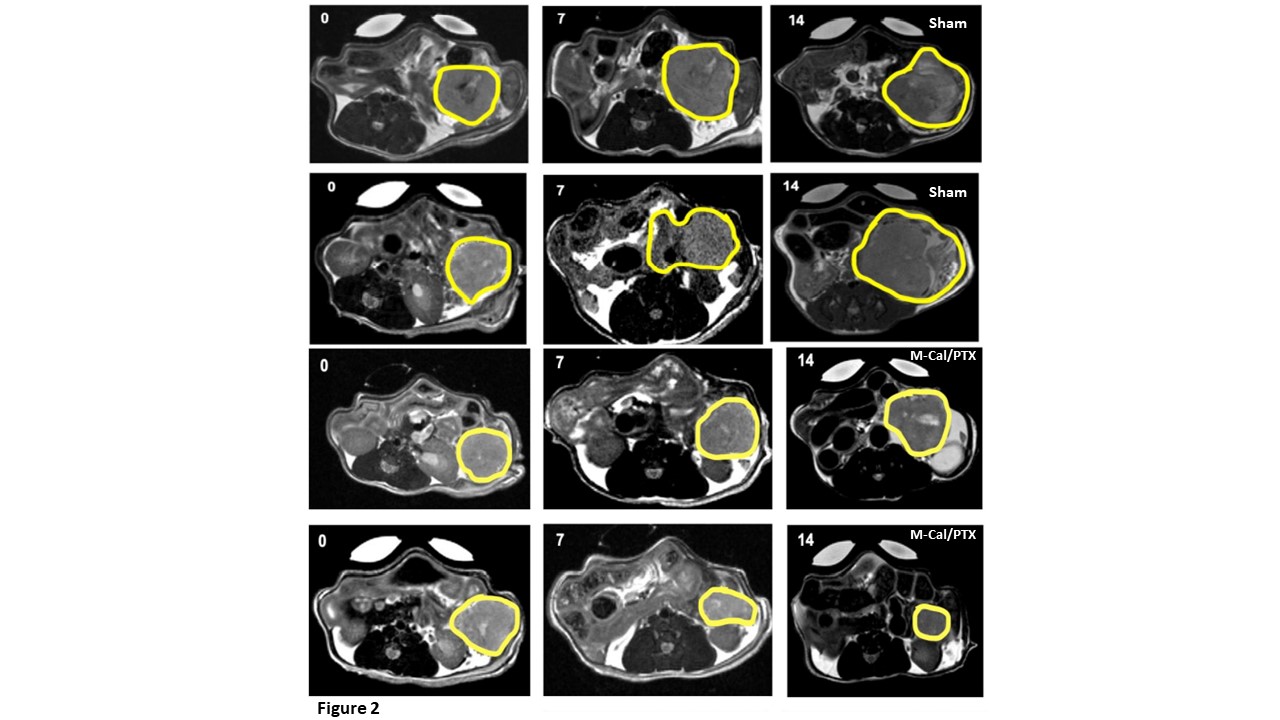
Figure 2. T2-MRI measurements of PDAC tumors in sham and M-Cal/PTX groups. Tumors were measured at baseline (day 0), midpoint (day 7) and on day 14.
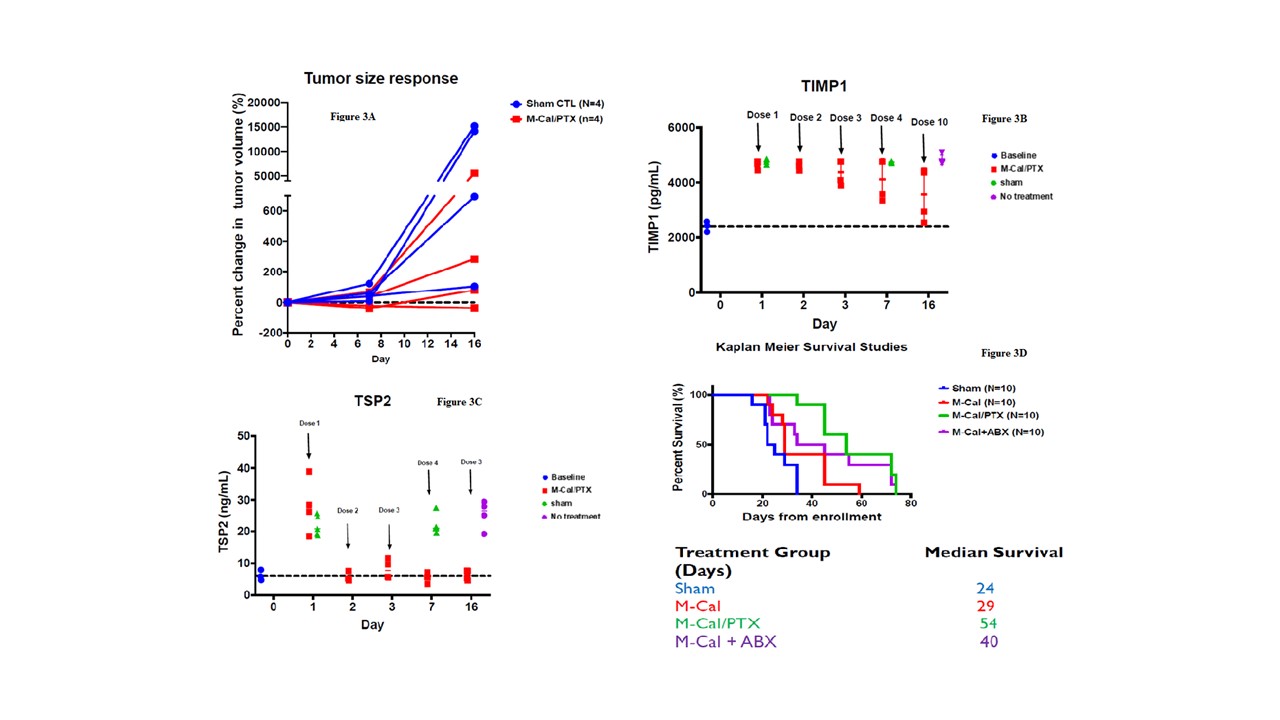
Figure 3. Change in tumor volumes with M-Cal/PTX (Red) and sham treatments (Blue) (A). Treatment effects on circulating stroma markers TIMP1(B) and TSP2 (C). Kaplan-Meier survival curves for mouse survival after various treatments (D).
Discovery and Basic Research - Pharmacology
Category: Poster Abstract
(W0930-05-27) Pharmacodynamic Assessments of Paclitaxel in Combination with Calcipotriol in Pancreatic Ductal Adenocarcinoma (PDAC)
Wednesday, October 19, 2022
9:30 AM – 10:30 AM ET

Fatima Dagher, MS
Research Assistant
University of Houston
Houston, United States- Vl
Victor R. lincha, Ph.D.
University of Houston
Houston, Texas, United States
Presenting Author(s)
Main Author(s)
Purpose: PDAC is an aggressive malignant neoplasm which continues to carry very poor prognosis despite advances in therapies over the past decade. The PDAC stroma-tumor microenvironment contributes to resistance mechanisms for optimum drug delivery and antitumor activity. Calcipotriol (Cal) is a stroma modulator, that is hypothesized to enable better drug delivery of paclitaxel (PTX) into the tumor tissues and enhance its cancer cell cytolytic activity. We recently developed and validated a UPLC-MS/MS assay for simultaneous quantifications of Cal and PTX at LLOQ of 0.5 ng/ml and developed a nano-polymeric micelle drug delivery system, loaded with Cal and PTX (M-Cal/PTX), for treatment of PDAC. Biodistribution studies using the micellar system confirmed Cal/PTX accumulation in orthotopic mouse PDAC models. The pharmacodynamics (efficacy) and safety assessments of M-Cal/PTX in the mouse PDAC model are presented herein.
Methods: To perform short-term efficacy, and safety studies, we used orthotopic Kras* mouse model of PDAC. The primary endpoints were to assess: 1) tumor response as measured by T2-MRI; and 2) the change in circulating stroma markers with M-Cal/PTX treatment. Mice received IV doses of M-Cal/PTX (0.5 mg/kg Cal and 5 mg/kg PTX); the dosing scheme used is summarized in Figure 1. Mono formulation of Cal (M-Cal) (N=3), a mixture of mono-formulated Cal and mono-formulated PTX (M-Cal + M-PTX) (N=4), a combination of M-Cal and Abraxane (M-Cal + ABX) (N=3) and a cremophor formulation of Cal and PTX (Crem-Cal + PTX) (N=6) were included as controls. The tumor size was measured by means of T2-MRI and the corresponding volumetric quantifications were obtained. The effects of treatment on the circulating levels of TIMP1, TSP2 and MMP7 were evaluated by ELISA. A survival study was conducted to estimate the treatment benefit in terms of overall survival.
Results: In the M-Cal/PTX group, there was a 75% response and one animal had significant tumor progression. In contrast, 75% of mice in the sham group showed tumor progression (Figure 2). Similarly, the M-Cal/PTX treatment benefit was demonstrated by assessing tumor volume (Figure 3A). Two animals in the M-Cal/PTX group showed a moderate increase in their tumor volumes from day 7 while one had tumor shrinkage at days 7 and 14. None of the animals in the sham group has tumor shrinkage. The effects of M-Cal/PTX on circulating plasma markers of stroma activity were measured. For TIMP1 a delayed response was observed (Figure 3B). The mean TIMP1 levels continued to decrease after the 4th dose (day 7) while the levels remained very high in the sham group. The effect of M-Cal/PTX on circulating TSP2 levels was rapid (Figure 3C). Reduction of TSP2 to baseline levels was achieved after the second dose and the levels remained low through the 10th dose. M-Cal/PTX did not impact the circulating levels of MMP7 after 10 doses. Treatment with M-Cal/PTX caused significant decrease on alpha-smooth muscle actin (𝛼-SMA), another reliable marker for stromal activity, compared with the sham group. We looked at the Ki-67 (a proliferation marker) and matrix protein collagen, and both were significantly inhibited in the treatment group compared to the sham group. For survival analysis, animals that were administered M-Cal/PTX lived the longest (median of 54 days) compared to of 24 days for those received sham (Figure 3D). We finally examined the effects of treatment on body weight and other markers of organ health (albumin, liver enzymes, blood urea nitrogen, globulin, and total protein). No significant change in weight with M-Cal/PTX compared to the sham group, and no notable abnormalities in the laboratory markers in the treatment group.
Conclusion: Our data shows that M-Cal/PTX is a potentially effective combination regimen for treatment of PDAC. Compared to sham and other treatment interventions, M-Cal/PTX was associated with reduction of stroma-related markers, better antitumor activity, and improvement in median survival of tumor-bearing mice. Furthermore, no changes in mouse weight in the M-Cal/PTX group or notable laboratory abnormalities suggesting a tolerable safety profile for this combination therapy at the tested dose. Additional preclinical studies are in progress to further explore and develop this optimal regimen for PDAC treatment.
.jpg)
Figure 1. Dosing scheme for the evaluation of short-term benefit of M-Cal/PTX in a mouse model of PDAC

Figure 2. T2-MRI measurements of PDAC tumors in sham and M-Cal/PTX groups. Tumors were measured at baseline (day 0), midpoint (day 7) and on day 14.

Figure 3. Change in tumor volumes with M-Cal/PTX (Red) and sham treatments (Blue) (A). Treatment effects on circulating stroma markers TIMP1(B) and TSP2 (C). Kaplan-Meier survival curves for mouse survival after various treatments (D).
