Back
Purpose: Saccharomyces boulardii (Sb) is a probiotic yeast strain generally regarded as safe (GRAS) through oral administration (1). Recently, bioengineered Saccharomyces boulardii (BSb) has been developed to secrete therapeutic antibodies “in-situ” in the gut to target various gastrointestinal (GI) diseases, including microorganism infection, colitis, colon cancer, and more (Data partially published) (2). We expect to formulate BSb into an oral dosage form to facilitate future preclinical/clinical studies. However, oral formulation of BSb presents several challenges, one of which is cell viability, which can decrease during multiple manufacturing unit operations and over product shelf-life. Lyophilization is a common drying technology that can be used to improve BSb stability. The final dosage form meeting the target product profiles (TPPs) can be directly produced as a lyophilized product with no need for further processing; in addition, modern lyophilizers are designed to programmatically perform multi-stage commands. Thus, the manufacturing of oral biologics can be streamlined through automated lyophilization, and the viability loss due to harsh manufacturing processes can be minimized. In the second place, compared with many other drying methods, lyophilization theoretically gives the greatest desiccation, and thus reduces potential cellular processes during storage to achieve long-term preservation (3). Herein, we explored the feasibility and advantages of using a lyophilizer to desiccate therapeutic BSb cells. Also, we want to demonstrate our progress on formulation design and progress optimization to lyophilize BSb cells, and a lyophilization-based streamlined preparation of ready-to-use biologic products where BSb cells doesn’t undergo additional unit operations.
Methods: Drying methods: Lyophilization was performed using LabConco FreeZone -84C Benthtop Freeze Dryer, spray-drying was performed using Buchi-290 mini spray dryer, and fluidized-drying was performed using Bosch SolidLab 1 bench top fluid bed system. Preparation of BSb-dopped rodent food: 2018SX Teklad global 18% protein rodent pellets were milled using Quadro Comil, followed by mixing with BSb suspension together with 10% binder. Resulting wet mass was manually shaped and subjected to lyophilization to give ready-to-use products. Properties of dried BSb products: For each dried product, water residue and drying survivability were measured. Water residue was characterized by LabMaster-aw neo to give water activity (aw), and HB43 Halogen Moisture Analyzer to give moisture content. Drying survivability was measured using LUNA-FL™ yeast viability kit, which stains live/dead yeast cells with different fluorescent colors that can be further visualized by Cytation 3 cell imaging multi-mode reader for viability measurement.
Results: BSb strains desiccated by different drying methods: Four different BSb strains (#210, FZY40-H1, FZY12-G7-E1, and FZMYA06-16) were desiccated using three different drying methods: lyophilization, spray drying, and fluidized-bed drying. Viability of spray-dried strains are >50% except for FZMYA06-16, lyophilized strains showed viability around 40%, while fluidized-dried sample gave less than 30% viability (Fig. 1). Lyophilized samples gave water content < 2%, and water activity < 0.02. While spray-dried and fluidized-dried samples gave water content > 4% and water activity > 0.15. Lyophilization process optimization: To study the affects of cooling rate on BSb survivability during lyophilization, two BSb strains (FZY12-G7-E1 and FZMYA06-16) were allocated to different cryotubes and underwent different freezing rates (ultra rapid cooling in liquid nitrogen, fast cooling in -80 °C freezer, and slow cooling in freezing container with isopropanol bath. Fully solidified samples were immediately transferred to benchtop lyophilizer to desiccate. BSb survivability in the lyophilized products was accordingly assessed (Fig. 2). Process parameters including cooling rate, drying temperature, and desiccating duration, will be further optimized and are expected to be included later. Characterization of BSb-dopped rodent food: Lyophilized rodent food gave adequate hardness (breaking force > 60 N), which makes it compatible to laboratory mice racks to facilitate animal studies. Storage stability of lyophilized food is under study. Once proved to be stable under storage conditions, we will apply BSb-dopped rodent food to animal studies and quantify the amount of live BSb cells in rodent feces (2).
Conclusion: Compared to other drying methods to desiccate BSb, lyophilization results in lowest water residue with viability around 40%. In addition, lyophilization enables streamlined preparation of solid dosages with adequate hardness, avoiding further manufacturing operations. More studies will be conducted to optimize the lyophilization process to increase the viability and stability of BSb products.
References: [1] Czerucka, D., Piche, T., & Rampal, P. Review article: yeast as probiotics–Saccharomyces boulardii. Aliment Pharmacol. 2007; 26 (6): 767-78.
[2] Chen, K., Zhu, Y., Zhang, Y., Hamza, T., Yu, H., Saint Fleur, A., ... & Feng, H. (2020). A probiotic yeast-based immunotherapy against Clostridioides difficile infection. Science translational medicine, 12(567), eaax4905.
[3] Rockinger, U., Funk, M., & Winter, G. (2021). Current approaches of preservation of cells during (freeze-) drying. Journal of Pharmaceutical Sciences, 110(8), 2873-2893.
Acknowledgements: This work is funded by R01AI148357-01 (NIAID). We also thank Dr. Ashley Saint Fleur and Dr. Yongrong Zhang for assistance with cell fermentation and imaging.
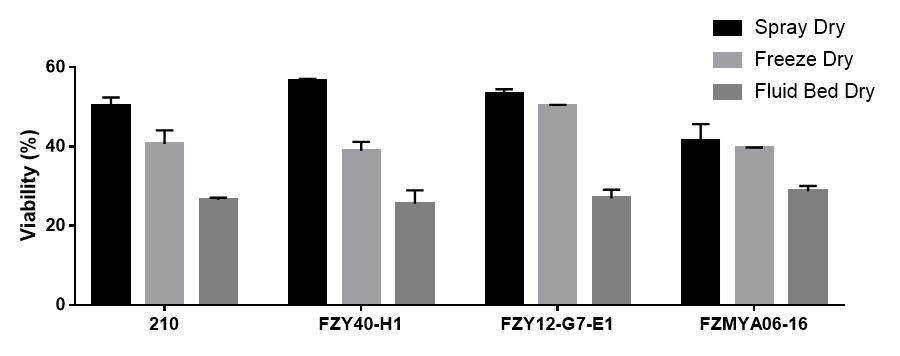
Figure 1. Viability of four different BSb strains after drying.
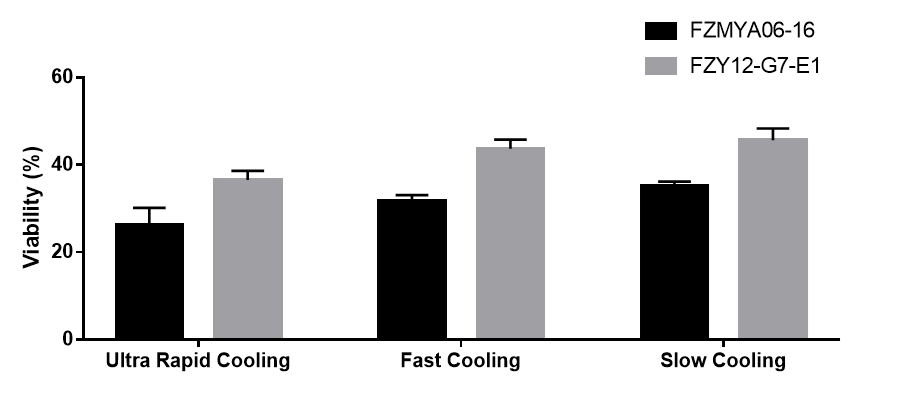
Figure 2. Cooling rate affects yeast viability after lyophilization for two BSb strains.
Formulation and Delivery - Biomolecular - Formulation
Category: Poster Abstract
(T1230-12-67) Stabilize and Formulate Yeast-Based Immunobiologics by Lyophilization for Oral Delivery
Tuesday, October 18, 2022
12:30 PM – 1:30 PM ET
- HC
Haixi Cui, BS
University of Maryland Baltimore
Baltimore, Maryland, United States - HC
Haixi Cui, BS
University of Maryland Baltimore
Baltimore, Maryland, United States
Presenting Author(s)
Main Author(s)
Purpose: Saccharomyces boulardii (Sb) is a probiotic yeast strain generally regarded as safe (GRAS) through oral administration (1). Recently, bioengineered Saccharomyces boulardii (BSb) has been developed to secrete therapeutic antibodies “in-situ” in the gut to target various gastrointestinal (GI) diseases, including microorganism infection, colitis, colon cancer, and more (Data partially published) (2). We expect to formulate BSb into an oral dosage form to facilitate future preclinical/clinical studies. However, oral formulation of BSb presents several challenges, one of which is cell viability, which can decrease during multiple manufacturing unit operations and over product shelf-life. Lyophilization is a common drying technology that can be used to improve BSb stability. The final dosage form meeting the target product profiles (TPPs) can be directly produced as a lyophilized product with no need for further processing; in addition, modern lyophilizers are designed to programmatically perform multi-stage commands. Thus, the manufacturing of oral biologics can be streamlined through automated lyophilization, and the viability loss due to harsh manufacturing processes can be minimized. In the second place, compared with many other drying methods, lyophilization theoretically gives the greatest desiccation, and thus reduces potential cellular processes during storage to achieve long-term preservation (3). Herein, we explored the feasibility and advantages of using a lyophilizer to desiccate therapeutic BSb cells. Also, we want to demonstrate our progress on formulation design and progress optimization to lyophilize BSb cells, and a lyophilization-based streamlined preparation of ready-to-use biologic products where BSb cells doesn’t undergo additional unit operations.
Methods: Drying methods: Lyophilization was performed using LabConco FreeZone -84C Benthtop Freeze Dryer, spray-drying was performed using Buchi-290 mini spray dryer, and fluidized-drying was performed using Bosch SolidLab 1 bench top fluid bed system. Preparation of BSb-dopped rodent food: 2018SX Teklad global 18% protein rodent pellets were milled using Quadro Comil, followed by mixing with BSb suspension together with 10% binder. Resulting wet mass was manually shaped and subjected to lyophilization to give ready-to-use products. Properties of dried BSb products: For each dried product, water residue and drying survivability were measured. Water residue was characterized by LabMaster-aw neo to give water activity (aw), and HB43 Halogen Moisture Analyzer to give moisture content. Drying survivability was measured using LUNA-FL™ yeast viability kit, which stains live/dead yeast cells with different fluorescent colors that can be further visualized by Cytation 3 cell imaging multi-mode reader for viability measurement.
Results: BSb strains desiccated by different drying methods: Four different BSb strains (#210, FZY40-H1, FZY12-G7-E1, and FZMYA06-16) were desiccated using three different drying methods: lyophilization, spray drying, and fluidized-bed drying. Viability of spray-dried strains are >50% except for FZMYA06-16, lyophilized strains showed viability around 40%, while fluidized-dried sample gave less than 30% viability (Fig. 1). Lyophilized samples gave water content < 2%, and water activity < 0.02. While spray-dried and fluidized-dried samples gave water content > 4% and water activity > 0.15. Lyophilization process optimization: To study the affects of cooling rate on BSb survivability during lyophilization, two BSb strains (FZY12-G7-E1 and FZMYA06-16) were allocated to different cryotubes and underwent different freezing rates (ultra rapid cooling in liquid nitrogen, fast cooling in -80 °C freezer, and slow cooling in freezing container with isopropanol bath. Fully solidified samples were immediately transferred to benchtop lyophilizer to desiccate. BSb survivability in the lyophilized products was accordingly assessed (Fig. 2). Process parameters including cooling rate, drying temperature, and desiccating duration, will be further optimized and are expected to be included later. Characterization of BSb-dopped rodent food: Lyophilized rodent food gave adequate hardness (breaking force > 60 N), which makes it compatible to laboratory mice racks to facilitate animal studies. Storage stability of lyophilized food is under study. Once proved to be stable under storage conditions, we will apply BSb-dopped rodent food to animal studies and quantify the amount of live BSb cells in rodent feces (2).
Conclusion: Compared to other drying methods to desiccate BSb, lyophilization results in lowest water residue with viability around 40%. In addition, lyophilization enables streamlined preparation of solid dosages with adequate hardness, avoiding further manufacturing operations. More studies will be conducted to optimize the lyophilization process to increase the viability and stability of BSb products.
References: [1] Czerucka, D., Piche, T., & Rampal, P. Review article: yeast as probiotics–Saccharomyces boulardii. Aliment Pharmacol. 2007; 26 (6): 767-78.
[2] Chen, K., Zhu, Y., Zhang, Y., Hamza, T., Yu, H., Saint Fleur, A., ... & Feng, H. (2020). A probiotic yeast-based immunotherapy against Clostridioides difficile infection. Science translational medicine, 12(567), eaax4905.
[3] Rockinger, U., Funk, M., & Winter, G. (2021). Current approaches of preservation of cells during (freeze-) drying. Journal of Pharmaceutical Sciences, 110(8), 2873-2893.
Acknowledgements: This work is funded by R01AI148357-01 (NIAID). We also thank Dr. Ashley Saint Fleur and Dr. Yongrong Zhang for assistance with cell fermentation and imaging.
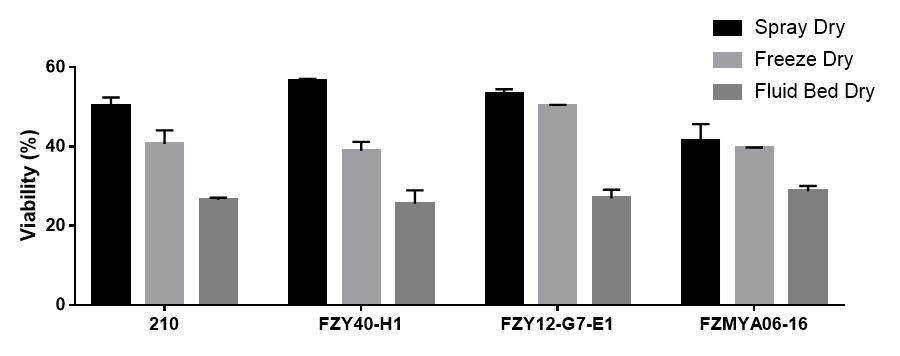
Figure 1. Viability of four different BSb strains after drying.
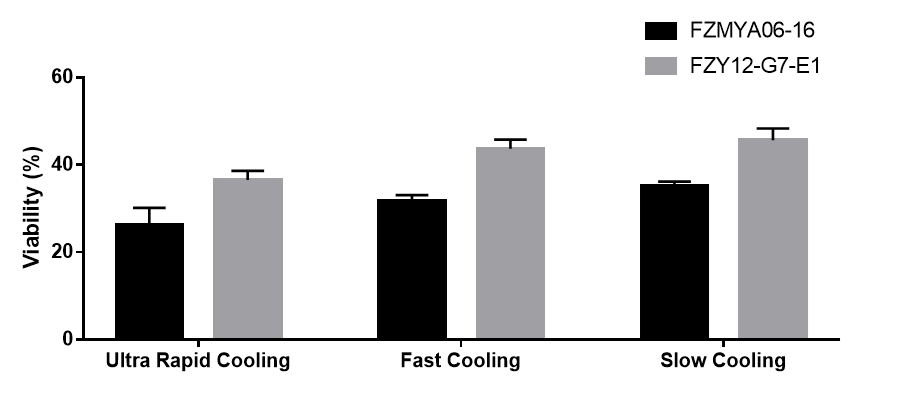
Figure 2. Cooling rate affects yeast viability after lyophilization for two BSb strains.
