Back
Purpose: Fixed-dose combination drug products combine two or more APIs in a single dosage form. Bilayer tablet technology is commonly used for FDC formulations to achieve desired therapeutic benefits; however, bilayer tablet formulations can present challenges such as lack of interfacial bonding, crack development, or layer separation [1]. The most common FDC formulations contain an immediate release (IR) low dose API and an extended-release (ER) mid to high dose API. If the IR layer API has good aqueous solubility, drug layering using a suitable film coating system over the ER tablet can overcome the bilayer formulation challenges. For drug layering, the selection of a suitable coating system along with optimized coating process parameters plays an important role in achieving good uniformity of the API on the tablet surface and within the production batch [2]. Sitagliptin and metformin HCl combination drug therapy is used in the treatment of Type 2 diabetic mellitus [3]. The purpose of this study was to develop a tablet formulation containing metformin HCl as an extended release (ER) tablet followed by drug layering of sitagliptin as an immediate release layer.
Methods: Metformin HCl ER tablets were manufactured using METHOCEL™ K100M as the rate controlling polymer with drug layering of sitagliptin using an Opadry® complete film coating system. Different coating process parameters were studied to explore the impact on tablet appearance and content uniformity (CU). The sitagliptin IR/metformin ER tablets were then film coated with Opadry® II High Performance Film Coating System then Opadry® EZ, Easy Swallow Film Coating System. (Table 1). Manufacturing Metformin HCl ER Tablets: While mixing constantly, a specified quantity of water was added to the MCC and the resulting wetted MCC then passed through ASTM #18 screen to further mix with the metformin, METHOCEL™ K100M and colloidal silicon dioxide in a DCM blender for 10 mins at 20 rpm. The powder blend was lubricated for an additional 2 minutes with magnesium stearate for 2 mins at 20 rpm. Tablets were compressed using a rotary tablet press using 21.2 x 10.6 mm, oval shaped, D-type standard concave tooling. The dissolution profile of uncoated tablets was obtained using USP Apparatus II with sinkers (100 rpm, 1000 mL phosphate buffer pH 6.8). Drug Layering of Sitagliptin Over Metformin HCl ER Tablets: Drug layering of sitagliptin over the metformin HCl ER tablets was performed using an Opadry coating system and the impact of various coating process parameters was studied, such as % solids, spray rate, and pan speed on tablet appearance and CU (Table 2). Coated tablets were evaluated for surface roughness, CU, and drug assay. Dissolution of drug layered sitagliptin tablets was obtained using USP Apparatus II (75 rpm, 900 mL, 0.1N HCl). Film coating of drug layered tablets: Sitagliptin-metformin FDC tablets were further film coated with Opadry II at 3% weight gain followed by Opadry EZ system at 1% weight gain.
Results: a. Metformin ER Tablet Physical Properties - The metformin ER formulation displayed satisfactory flow properties with good compressibility. Tablets showed good hardness ~24 kP and very low friability ~0.3 %. b. Drug Layering of Sitagliptin Over Metformin ER Tablets: - Drug layered sitagliptin-metformin HCl FDC tablets in Trials 1 and 2 (higher spray rate with 12.5% and 10% solids) produced tablets with a slightly rough surface; whereas, drug layered sitagliptin tablets prepared using Trial 3 (low spray rate and 10% solids) had a smooth surface (Figure 1). An additional coating trial (Trial 4) was performed on a 12 kg scale using an ACG Quest TCM machine with the process parameters based on Trial 3. The resulting Trial 4 coated tablets had a smooth surface appearance (Figure 1). c. Content Uniformity, Assay and Dissolution Testing for Sitagliptin: - Drug layered sitagliptin tablets from Trials 1 and 2 gave higher AV of 22 and 23, respectively for CU assay, indicating the nonuniform distribution of the drug. However, Trials 3 and 4, in which optimized coating process parameters were used (lower % solids, lower spray rate and higher pan speed) gave lower AV values ~11, indicating the uniform distribution of the drug. d. Assay and Dissolution Testing for Metformin HCl. - The assay content ~ 98.0% was obtained for the metformin ER tablet with no significant difference in release profile for uncoated, drug layered, and final film coated tablets.
Conclusion: Drug layering of IR sitagliptin (100 mg) over metformin ER tablets was successfully achieved with the use of an Opadry coating system along with optimization of coating process parameters to achieve a smooth uniform surface and acceptable CU. Film coating of tablets with Opadry II and Opadry EZ further improved tablets robustness without impacting release profiles of either drugs. More than 90% of sitagliptin was released in 45 minutes and the dissolution of metformin remained consistent.
References: 1. Mehul, Patel & Sockan, Ganesh & Mani, Tamizh. (2010). Challenges in the formulation of bilayered tablets: A review. Int J Pharma Res Dev. 2.
2. Wirges M, Funke A, Serno P, Knop K, Kleinebudde P. Monitoring of an active coating process for two-layer tablets-model development strategies. J Pharm Sci. 2013 Feb;102(2):556-64. doi: 10.1002/jps.23383. Epub 2012 Nov 27. PMID: 23188659.
3. Hayes, J., Anderson, R., & Stephens, J. W. (2016). Sitagliptin/metformin fixed-dose combination in type 2 diabetes mellitus: an evidence-based review of its place in therapy. Drug design, development, and therapy, 10, 2263–2270. https://doi.org/10.2147/DDDT.S93076

Table 1

Table 2
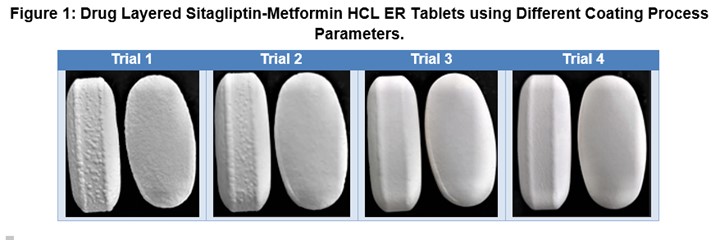
Figure 1
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(T1130-03-18) Impact of Coating Process Parameters on Drug Layering of Sitagliptin over Metformin HCl ER Tablets
Tuesday, October 18, 2022
11:30 AM – 12:30 PM ET
- MG
Manish Ghimire, Ph.D.
Colorcon, Inc.
Harleysville, Pennsylvania, United States - VA
Vaibhav Ambudkar, MS
Colorcon Asia Pvt. Limited
Goa, Goa, India
Presenting Author(s)
Main Author(s)
Purpose: Fixed-dose combination drug products combine two or more APIs in a single dosage form. Bilayer tablet technology is commonly used for FDC formulations to achieve desired therapeutic benefits; however, bilayer tablet formulations can present challenges such as lack of interfacial bonding, crack development, or layer separation [1]. The most common FDC formulations contain an immediate release (IR) low dose API and an extended-release (ER) mid to high dose API. If the IR layer API has good aqueous solubility, drug layering using a suitable film coating system over the ER tablet can overcome the bilayer formulation challenges. For drug layering, the selection of a suitable coating system along with optimized coating process parameters plays an important role in achieving good uniformity of the API on the tablet surface and within the production batch [2]. Sitagliptin and metformin HCl combination drug therapy is used in the treatment of Type 2 diabetic mellitus [3]. The purpose of this study was to develop a tablet formulation containing metformin HCl as an extended release (ER) tablet followed by drug layering of sitagliptin as an immediate release layer.
Methods: Metformin HCl ER tablets were manufactured using METHOCEL™ K100M as the rate controlling polymer with drug layering of sitagliptin using an Opadry® complete film coating system. Different coating process parameters were studied to explore the impact on tablet appearance and content uniformity (CU). The sitagliptin IR/metformin ER tablets were then film coated with Opadry® II High Performance Film Coating System then Opadry® EZ, Easy Swallow Film Coating System. (Table 1). Manufacturing Metformin HCl ER Tablets: While mixing constantly, a specified quantity of water was added to the MCC and the resulting wetted MCC then passed through ASTM #18 screen to further mix with the metformin, METHOCEL™ K100M and colloidal silicon dioxide in a DCM blender for 10 mins at 20 rpm. The powder blend was lubricated for an additional 2 minutes with magnesium stearate for 2 mins at 20 rpm. Tablets were compressed using a rotary tablet press using 21.2 x 10.6 mm, oval shaped, D-type standard concave tooling. The dissolution profile of uncoated tablets was obtained using USP Apparatus II with sinkers (100 rpm, 1000 mL phosphate buffer pH 6.8). Drug Layering of Sitagliptin Over Metformin HCl ER Tablets: Drug layering of sitagliptin over the metformin HCl ER tablets was performed using an Opadry coating system and the impact of various coating process parameters was studied, such as % solids, spray rate, and pan speed on tablet appearance and CU (Table 2). Coated tablets were evaluated for surface roughness, CU, and drug assay. Dissolution of drug layered sitagliptin tablets was obtained using USP Apparatus II (75 rpm, 900 mL, 0.1N HCl). Film coating of drug layered tablets: Sitagliptin-metformin FDC tablets were further film coated with Opadry II at 3% weight gain followed by Opadry EZ system at 1% weight gain.
Results: a. Metformin ER Tablet Physical Properties - The metformin ER formulation displayed satisfactory flow properties with good compressibility. Tablets showed good hardness ~24 kP and very low friability ~0.3 %. b. Drug Layering of Sitagliptin Over Metformin ER Tablets: - Drug layered sitagliptin-metformin HCl FDC tablets in Trials 1 and 2 (higher spray rate with 12.5% and 10% solids) produced tablets with a slightly rough surface; whereas, drug layered sitagliptin tablets prepared using Trial 3 (low spray rate and 10% solids) had a smooth surface (Figure 1). An additional coating trial (Trial 4) was performed on a 12 kg scale using an ACG Quest TCM machine with the process parameters based on Trial 3. The resulting Trial 4 coated tablets had a smooth surface appearance (Figure 1). c. Content Uniformity, Assay and Dissolution Testing for Sitagliptin: - Drug layered sitagliptin tablets from Trials 1 and 2 gave higher AV of 22 and 23, respectively for CU assay, indicating the nonuniform distribution of the drug. However, Trials 3 and 4, in which optimized coating process parameters were used (lower % solids, lower spray rate and higher pan speed) gave lower AV values ~11, indicating the uniform distribution of the drug. d. Assay and Dissolution Testing for Metformin HCl. - The assay content ~ 98.0% was obtained for the metformin ER tablet with no significant difference in release profile for uncoated, drug layered, and final film coated tablets.
Conclusion: Drug layering of IR sitagliptin (100 mg) over metformin ER tablets was successfully achieved with the use of an Opadry coating system along with optimization of coating process parameters to achieve a smooth uniform surface and acceptable CU. Film coating of tablets with Opadry II and Opadry EZ further improved tablets robustness without impacting release profiles of either drugs. More than 90% of sitagliptin was released in 45 minutes and the dissolution of metformin remained consistent.
References: 1. Mehul, Patel & Sockan, Ganesh & Mani, Tamizh. (2010). Challenges in the formulation of bilayered tablets: A review. Int J Pharma Res Dev. 2.
2. Wirges M, Funke A, Serno P, Knop K, Kleinebudde P. Monitoring of an active coating process for two-layer tablets-model development strategies. J Pharm Sci. 2013 Feb;102(2):556-64. doi: 10.1002/jps.23383. Epub 2012 Nov 27. PMID: 23188659.
3. Hayes, J., Anderson, R., & Stephens, J. W. (2016). Sitagliptin/metformin fixed-dose combination in type 2 diabetes mellitus: an evidence-based review of its place in therapy. Drug design, development, and therapy, 10, 2263–2270. https://doi.org/10.2147/DDDT.S93076

Table 1

Table 2

Figure 1
