Back
Purpose: Poorly water-soluble drugs exhibit limited and often unpredictable intestinal absorption that can be improved with co-administration of lipid-based formulations that stimulate mesenteric lymphatic transport [1]. Lymph duct cannulated animal models remain the only reliable means to assess intestinal lymphatic absorption pre-clinically as colonocyte-like lipid metabolism in the immortalized Caco-2 culture model has hindered the development of in vitro-in vivo correlations from lipoprotein-associated drug transport studies [2]. Primary small intestinal monolayers that retain the functionality of native enterocytes may help overcome this challenge and serve as an in vitro screening tool to assess lymphatic absorption [3].
Methods: Lipoprotein secretion from primary human duodenum and ileum monolayers was stimulated with mixed micelles containing oleic acid (OA) or inhibited with a microsomal triglyceride transfer protein (MTP) inhibitor (lomitapide/Juxtapid®), and the results compared to Caco-2. The effect of 2-monoolein (2-MO) on lipoprotein secretion was tested to examine the 2-monoglyceride re-esterification pathway. A novel assay workflow, based on protocols optimized for Caco-2 [4]–[6], was developed to accommodate primary cell culture and enable direct comparison to Caco-2. Chylomicron (CM) and very-low density lipoprotein (VLDL) secretion was assessed by Western blotting of apolipoprotein-B48 (apoB-48) and apoB-100, respectively. Secreted lipoprotein fractions isolated by density-gradient ultracentrifugation were analyzed for apoB and triglyceride (TG) content using a sandwich ELISA and a colorimetric assay, respectively. We hypothesized that 2-MO would stimulate greater TG secretion from primary cells than Caco-2, potentially providing a platform to test lymphatic transport of lipophilic drugs that are suspected to associate with CMs generated in the fed-state.
Results: Caco-2 secreted apoB-48 and apoB-100 in the apical and basolateral directions following lipid stimulation whereas primary monolayers appeared to secrete apoB-48 in the basolateral direction exclusively. Following lipid stimulation with 2-MO, TG content in secreted fractions containing both CMs and VLDLs were elevated 3-fold (duodenum), 5-fold (ileum) and 1.5-fold (Caco-2) compared to lipid stimulation without 2-MO. MTP inhibition significantly decreased apoB secretion from Caco-2, but not from the primary cells, and no effect of MTP inhibition on TG output was observed in any of the cell types (compared to control conditions where monolayers received medium). Primary cells secreted more than 100 times less total apoB in isolated CM and VLDL fractions than Caco-2.
Conclusion: Our results indicate that primary duodenum and ileum monolayers secrete mostly CMs in the basolateral direction upon lipid stimulation whereas Caco-2 secrete mostly VLDLs in both the apical and basolateral directions. Moreover, the 2-MG re-esterification pathway appears dominant in primary small intestinal cells compared to Caco-2, which agrees with the literature and our hypothesis [2], [7]. The strikingly greater ratio of TG to apoB in secreted lipoproteins from primary cells (1.5-3 mg TG/ng apoB) compared to Caco-2 (~0.003 mg TG/ng apoB) indicates higher TG load per secreted lipoprotein particle, in line with the large CMs known to be produced by the post-prandial small intestine. The workflow established herein is therefore promising as a lymphatic absorption screening tool, which we plan to assess next via apical-basolateral lipoprotein-associated transport studies with model compounds known to undergo oral lymphatic transport.
References: [1] N. L. Trevaskis, W. N. Charman, and C. J. H. Porter, “Lipid-based delivery systems and intestinal lymphatic drug transport: A mechanistic update,” Adv. Drug Deliv. Rev., vol. 60, no. 6, pp. 702–716, 2008.
[2] A. M. Nauli, Y. Sun, J. D. Whittimore, S. Atyia, G. Krishnaswamy, and S. M. Nauli, “Chylomicrons produced by Caco-2 cells contained ApoB-48 with diameter of 80-200 nm,” Physiol. Rep., vol. 2, no. 6, pp. 1–12, 2014.
[3] J. E. Speer et al., “Molecular transport through primary human small intestinal monolayers by culture on a collagen scaffold with a gradient of chemical cross-linking,” J. Biol. Eng., vol. 13, no. 1, pp. 1–15, 2019.
[4] J. Luchoomun, “Assembly and Secretion of Chylomicrons by Differentiated Caco-2 cells,” J. Biol. Chem., vol. 274, no. 28, pp. 19565–19572, 1999.
[5] D. Chateau, T. Pauquai, F. Delers, M. Rousset, J. Chambaz, and S. Demignot, “Lipid micelles stimulate the secretion of triglyceride-enriched apolipoprotein B48-containing lipoproteins by Caco-2 cells,” J. Cell. Physiol., vol. 202, no. 3, pp. 767–776, 2005.
[6] A. M. Nauli and J. D. Whittimore, “Using caco-2 cells to study lipid transport by the intestine,” J. Vis. Exp., vol. 2015, no. 102, pp. 1–8, 2015.
[7] D. Venugopal and J. I. Godon, “Trafficking of exogenous fatty acids within Caco2 cells,” J. Lipid Res., vol. 33, pp. 9–19, 1992.
Acknowledgements:This work was completed in collaboration with the Novartis Institutes for BioMedical Research, Inc.
.jpg)
Figure 1. Assay workflow. Red text indicates next steps in which apical-basolateral lipoprotein-associated drug transport will be compared to data from lymph duct cannulated mice models receiving analagous inhibition or stimulation.
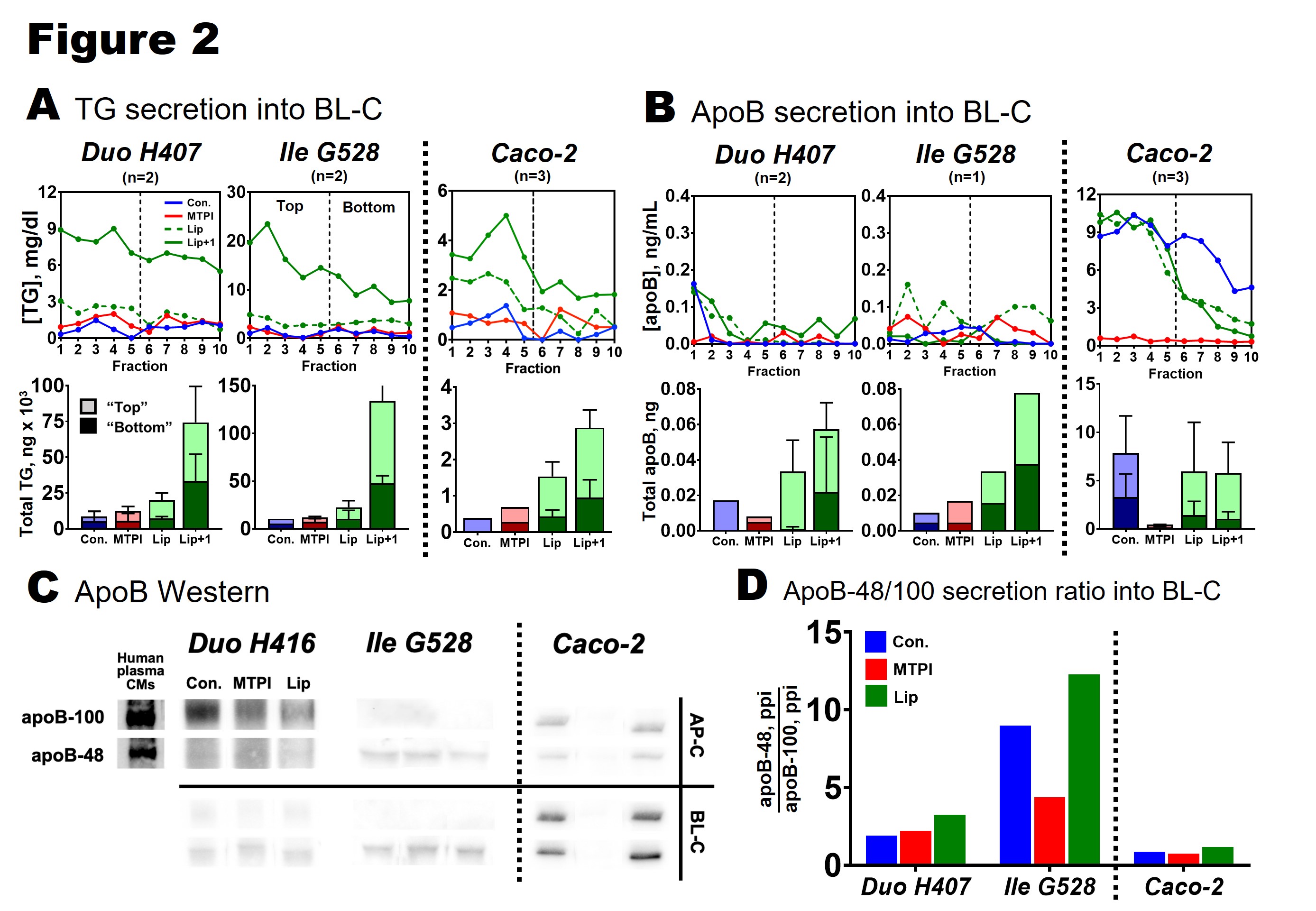
Figure 2. Lipoprotein secretion from primary duodenum (Duo H407), primary ileum (Ile G528), and Caco-2 monolayers. A: TG secretion into the basolateral compartment (BL-C). Fractions 1-10 (each 100 uL) represent isolated CMs and VLDLs (Svedberg floatation coefficient ≥ 20). Bars show total TG in the top 5 fractions ("top," light shading) and bottom 5 fractions ("bottom," dark shading). All data was corrected for TG contribution from medium. B: apoB secretion into the BL-C. All data was corrected for apoB contribution from medium. C: apoB Western blots for each cell type. Positive control supplied by CMs purified from human plasma. ApoB secreted into the AP-C and BL-C was immunoprecipated and blotted on the same gel (cell types blotted separately). D: ApoB isoform secretion ratio into BL-C calculated from Western blot in C. ApoB-48 signal (ppi) was divided by apoB-100 signal (ppi) for each treatment.
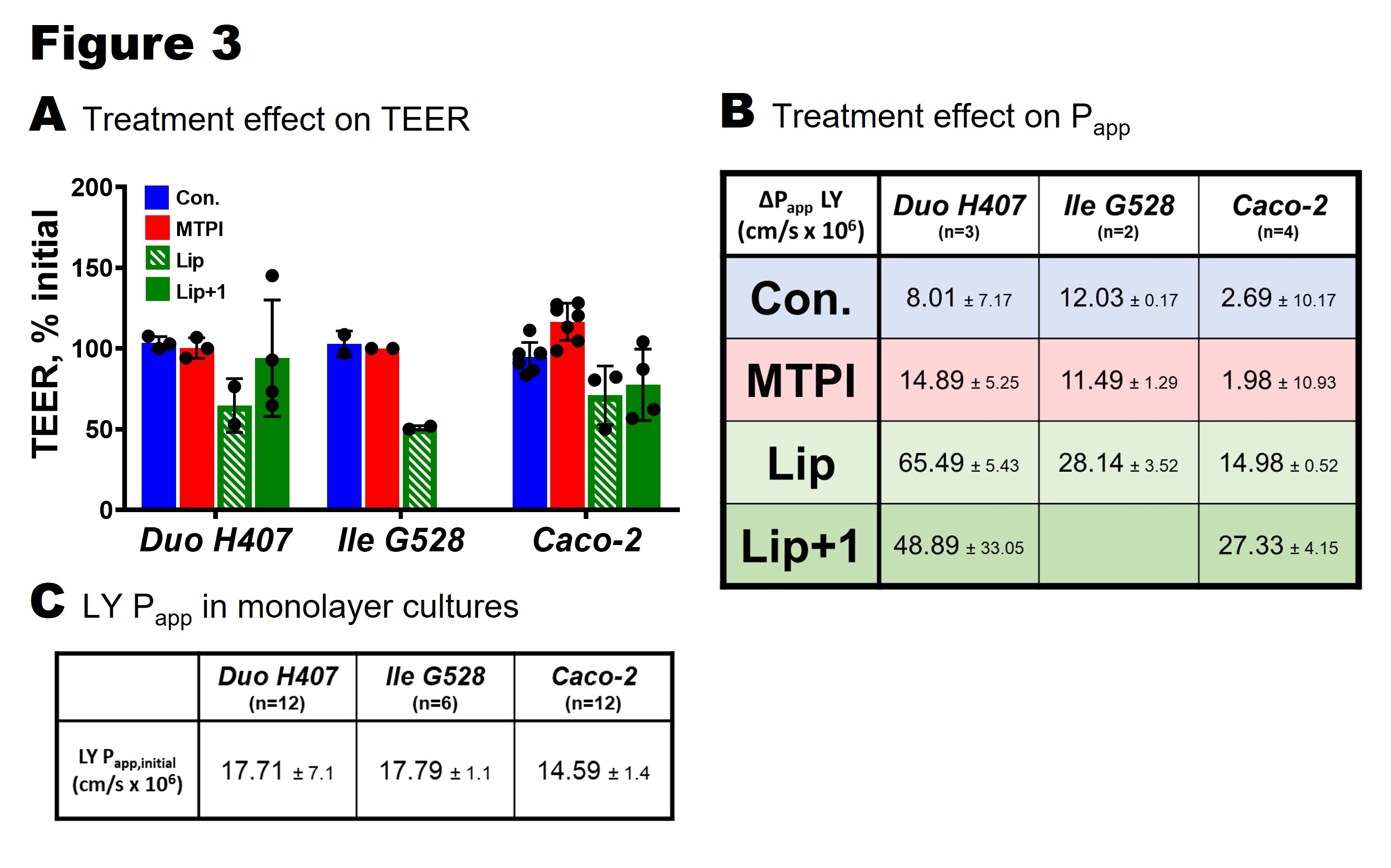
Figure 3. Effect of treatments on barrier integrity of primary duodenum (Duo H407), primary ileum (Ile G528), and Caco-2 monolayers. A. Change in TEER from 4 hour treatments calculated by measuring TEER before and after exposure. B: Change in Lucifer yellow (LY) permeability (apical-basolateral) after 4 hours of treatment. Change in Papp calculated by subtracting initial measurements (Papp,initial) from final measurements (Papp,final). C: Initial LY Papp for each cell type (representing normal, healthy monolayer functionality).
Preclinical Development - Biomolecular - ADME
Category: Poster Abstract
(T1130-02-08) In Vitro Lymphatic Absorption Screening with Primary Human Small Intestinal Cells
Tuesday, October 18, 2022
11:30 AM – 12:30 PM ET
- IS
Ian M. Smith
PhD Candidate
Northeastern University
Boston, Massachusetts, United States - RC
Rebecca L. Carrier, Ph.D.
Northeastern University
Boston, Massachusetts, United States
Presenting Author(s)
Main Author(s)
Purpose: Poorly water-soluble drugs exhibit limited and often unpredictable intestinal absorption that can be improved with co-administration of lipid-based formulations that stimulate mesenteric lymphatic transport [1]. Lymph duct cannulated animal models remain the only reliable means to assess intestinal lymphatic absorption pre-clinically as colonocyte-like lipid metabolism in the immortalized Caco-2 culture model has hindered the development of in vitro-in vivo correlations from lipoprotein-associated drug transport studies [2]. Primary small intestinal monolayers that retain the functionality of native enterocytes may help overcome this challenge and serve as an in vitro screening tool to assess lymphatic absorption [3].
Methods: Lipoprotein secretion from primary human duodenum and ileum monolayers was stimulated with mixed micelles containing oleic acid (OA) or inhibited with a microsomal triglyceride transfer protein (MTP) inhibitor (lomitapide/Juxtapid®), and the results compared to Caco-2. The effect of 2-monoolein (2-MO) on lipoprotein secretion was tested to examine the 2-monoglyceride re-esterification pathway. A novel assay workflow, based on protocols optimized for Caco-2 [4]–[6], was developed to accommodate primary cell culture and enable direct comparison to Caco-2. Chylomicron (CM) and very-low density lipoprotein (VLDL) secretion was assessed by Western blotting of apolipoprotein-B48 (apoB-48) and apoB-100, respectively. Secreted lipoprotein fractions isolated by density-gradient ultracentrifugation were analyzed for apoB and triglyceride (TG) content using a sandwich ELISA and a colorimetric assay, respectively. We hypothesized that 2-MO would stimulate greater TG secretion from primary cells than Caco-2, potentially providing a platform to test lymphatic transport of lipophilic drugs that are suspected to associate with CMs generated in the fed-state.
Results: Caco-2 secreted apoB-48 and apoB-100 in the apical and basolateral directions following lipid stimulation whereas primary monolayers appeared to secrete apoB-48 in the basolateral direction exclusively. Following lipid stimulation with 2-MO, TG content in secreted fractions containing both CMs and VLDLs were elevated 3-fold (duodenum), 5-fold (ileum) and 1.5-fold (Caco-2) compared to lipid stimulation without 2-MO. MTP inhibition significantly decreased apoB secretion from Caco-2, but not from the primary cells, and no effect of MTP inhibition on TG output was observed in any of the cell types (compared to control conditions where monolayers received medium). Primary cells secreted more than 100 times less total apoB in isolated CM and VLDL fractions than Caco-2.
Conclusion: Our results indicate that primary duodenum and ileum monolayers secrete mostly CMs in the basolateral direction upon lipid stimulation whereas Caco-2 secrete mostly VLDLs in both the apical and basolateral directions. Moreover, the 2-MG re-esterification pathway appears dominant in primary small intestinal cells compared to Caco-2, which agrees with the literature and our hypothesis [2], [7]. The strikingly greater ratio of TG to apoB in secreted lipoproteins from primary cells (1.5-3 mg TG/ng apoB) compared to Caco-2 (~0.003 mg TG/ng apoB) indicates higher TG load per secreted lipoprotein particle, in line with the large CMs known to be produced by the post-prandial small intestine. The workflow established herein is therefore promising as a lymphatic absorption screening tool, which we plan to assess next via apical-basolateral lipoprotein-associated transport studies with model compounds known to undergo oral lymphatic transport.
References: [1] N. L. Trevaskis, W. N. Charman, and C. J. H. Porter, “Lipid-based delivery systems and intestinal lymphatic drug transport: A mechanistic update,” Adv. Drug Deliv. Rev., vol. 60, no. 6, pp. 702–716, 2008.
[2] A. M. Nauli, Y. Sun, J. D. Whittimore, S. Atyia, G. Krishnaswamy, and S. M. Nauli, “Chylomicrons produced by Caco-2 cells contained ApoB-48 with diameter of 80-200 nm,” Physiol. Rep., vol. 2, no. 6, pp. 1–12, 2014.
[3] J. E. Speer et al., “Molecular transport through primary human small intestinal monolayers by culture on a collagen scaffold with a gradient of chemical cross-linking,” J. Biol. Eng., vol. 13, no. 1, pp. 1–15, 2019.
[4] J. Luchoomun, “Assembly and Secretion of Chylomicrons by Differentiated Caco-2 cells,” J. Biol. Chem., vol. 274, no. 28, pp. 19565–19572, 1999.
[5] D. Chateau, T. Pauquai, F. Delers, M. Rousset, J. Chambaz, and S. Demignot, “Lipid micelles stimulate the secretion of triglyceride-enriched apolipoprotein B48-containing lipoproteins by Caco-2 cells,” J. Cell. Physiol., vol. 202, no. 3, pp. 767–776, 2005.
[6] A. M. Nauli and J. D. Whittimore, “Using caco-2 cells to study lipid transport by the intestine,” J. Vis. Exp., vol. 2015, no. 102, pp. 1–8, 2015.
[7] D. Venugopal and J. I. Godon, “Trafficking of exogenous fatty acids within Caco2 cells,” J. Lipid Res., vol. 33, pp. 9–19, 1992.
Acknowledgements:This work was completed in collaboration with the Novartis Institutes for BioMedical Research, Inc.
.jpg)
Figure 1. Assay workflow. Red text indicates next steps in which apical-basolateral lipoprotein-associated drug transport will be compared to data from lymph duct cannulated mice models receiving analagous inhibition or stimulation.

Figure 2. Lipoprotein secretion from primary duodenum (Duo H407), primary ileum (Ile G528), and Caco-2 monolayers. A: TG secretion into the basolateral compartment (BL-C). Fractions 1-10 (each 100 uL) represent isolated CMs and VLDLs (Svedberg floatation coefficient ≥ 20). Bars show total TG in the top 5 fractions ("top," light shading) and bottom 5 fractions ("bottom," dark shading). All data was corrected for TG contribution from medium. B: apoB secretion into the BL-C. All data was corrected for apoB contribution from medium. C: apoB Western blots for each cell type. Positive control supplied by CMs purified from human plasma. ApoB secreted into the AP-C and BL-C was immunoprecipated and blotted on the same gel (cell types blotted separately). D: ApoB isoform secretion ratio into BL-C calculated from Western blot in C. ApoB-48 signal (ppi) was divided by apoB-100 signal (ppi) for each treatment.

Figure 3. Effect of treatments on barrier integrity of primary duodenum (Duo H407), primary ileum (Ile G528), and Caco-2 monolayers. A. Change in TEER from 4 hour treatments calculated by measuring TEER before and after exposure. B: Change in Lucifer yellow (LY) permeability (apical-basolateral) after 4 hours of treatment. Change in Papp calculated by subtracting initial measurements (Papp,initial) from final measurements (Papp,final). C: Initial LY Papp for each cell type (representing normal, healthy monolayer functionality).
