Back
Purpose: Development of stable multivitamin formulation is always challenging due to inherent degradation potential of vitamins. Currently, U.S. law requires that all fortified foods, including dietary supplements must contain, at minimum, 100% of the label-claimed amount of nutrients throughout the shelf life of the product. Therefore, to maintain the 100% label claim, overages must be added to the dietary supplements. The purpose of this study is to develop the robust chewable tablets containing vitamin A, D, E, and K and to determine their overages used to maintain the 100% label claim throughout the shelf life of the products. Hardness of chewable tablets is a critical quality attribute, and it must have optimum hardness to chew without causing damage to dentures. For stability evaluation, ASAPprime® study was performed, and the overages were determined and compared with the stability data collected at accelerated and room temperature conditions. In order to improve the palatability and patient compliance, sweetener, and flavors were added to the formula.
Methods: A mix of vitamins, minerals, and herbals was procured and mixed with excipients after screening through mesh # 20. Mixing was carried out in a V-Shell blender and finally the mix was compressed. Hardness of tablets were determined by keeping the tablets horizontally between the platens and the force required to break the tablets was recorded and later evaluated for case hardening. To ensure the uniformity of vitamins and minerals in the finished product, stratified sampling was performed during the compression of scale-up batch. Vit C and zinc were tested to ensure the uniformity of active components in the mix. Vit A constitutes 0.02% in the finished product, and therefore samples collected at start, middle, and end of compression were tested to ensure the uniformity. ASAPprime® study was performed to predict the overages used for vitamins in the formula and to get the information on final package required to maintain the shelf of finished product. ASAPprime® study consists of 21-day protocol, in which the samples are exposed to extreme temperature and humidity conditions and the associated software utilizes the Monte Carlo simulations to predict shelf life.
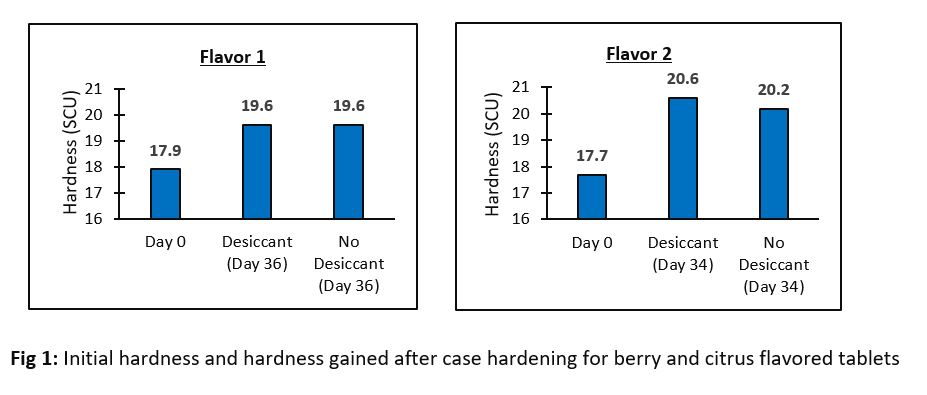
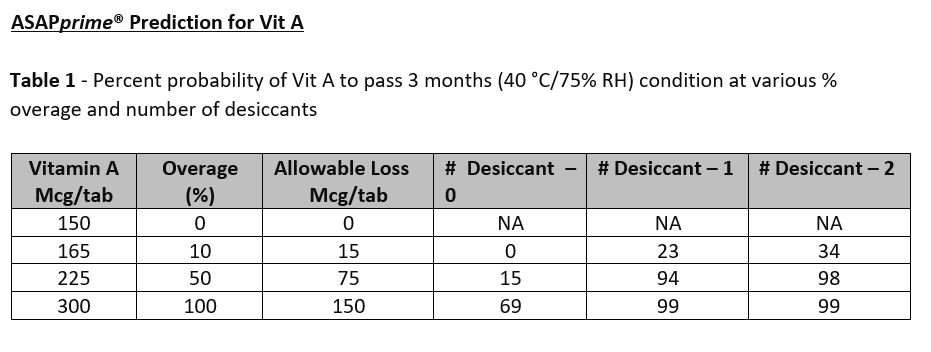
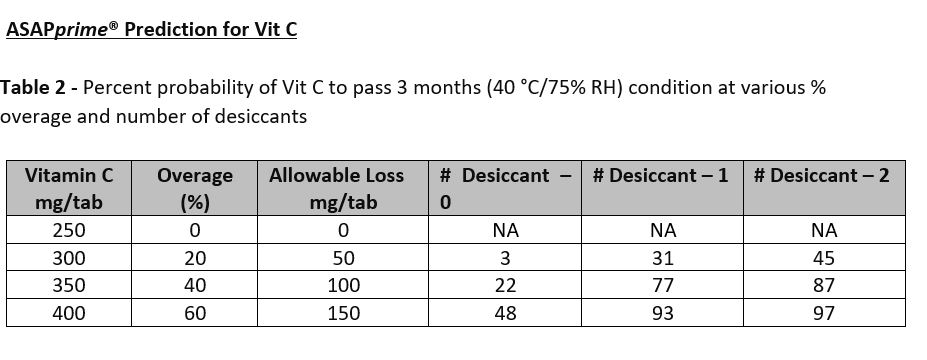
Results: The compressed tablet consisted of a mix of vitamins, minerals, and herbal component faced challenges during the compression. Black spots were observed on the tablets during the compression and a remedial measure was taken by using (n-1) strategy in which each component of formula was removed at a time to ascertain the causative excipient responsible for black spots. It was found that the magnesium oxide added to the formula to provide magnesium was responsible for black spots and later replaced with magnesium sulfate. We also found that the tablets undergo case hardening and gained hardness post-compression by 2-4 SCU. The target hardness set was 17.5 SCU and was comparable to reference product. Flavor concentration was evaluated and found that the concentration of 3.346% was optimal in the final formula. To ensure uniformity of actives in finished product, a total of 30 samples were collected during the run and tested for Vit C and Zinc and our results confirmed the uniformity of vitamins and minerals in the finished product. Compressed tablets were also tested for Vit A and our results showed uniform distribution of Vit A in the finished product. The results of ASAPprime® study showed that Vit A and Vit C undergo extensive degradation. Table 1 below for Vit A showed the predicted overage of 100% and one desiccant in the packaging container required to pass 3 months accelerated condition with a probability of 99%. The accelerated condition data generated for Vit A (data not shown) were in agreement with the predictions of ASAPprime® study. However, for Vit C, table 2 below showed the predicted overage of 60% and 2 desiccants in the final container required to pass 3 months accelerated condition with a probability of 97%. In contrast, our results for Vit C at accelerated conditions passed the 3 months using an overage of 35% and one desiccant. The ongoing stability results for Vit C in room temperature conditions (data not shown) is similar to the results obtained at accelerated conditions.
Conclusion: Our studies conclude that hardness of developed multivitamin chewable tablets was comparable to reference product. Among the vitamins, Vit D and Vit E did not undergo much degradation in the ASAPprime® study and accelerated temperature and humidity conditions. Predicted data by ASAPprime® study was correlated well with accelerated data of Vit A but not for Vit C, and thus warrants conducting the stability studies in accelerated and room temperature conditions.
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(T1030-04-19) Formulation Development of Dietary Supplements and Stability Prediction Using Accelerated Stability Assessment Program
Tuesday, October 18, 2022
10:30 AM – 11:30 AM ET

Amit Bansal, PhD
Scientist II
Perrigo
Kalamazoo, Michigan, United States
Amit Bansal, PhD
Scientist II
Perrigo
Kalamazoo, Michigan, United States
Presenting Author(s)
Main Author(s)
Purpose: Development of stable multivitamin formulation is always challenging due to inherent degradation potential of vitamins. Currently, U.S. law requires that all fortified foods, including dietary supplements must contain, at minimum, 100% of the label-claimed amount of nutrients throughout the shelf life of the product. Therefore, to maintain the 100% label claim, overages must be added to the dietary supplements. The purpose of this study is to develop the robust chewable tablets containing vitamin A, D, E, and K and to determine their overages used to maintain the 100% label claim throughout the shelf life of the products. Hardness of chewable tablets is a critical quality attribute, and it must have optimum hardness to chew without causing damage to dentures. For stability evaluation, ASAPprime® study was performed, and the overages were determined and compared with the stability data collected at accelerated and room temperature conditions. In order to improve the palatability and patient compliance, sweetener, and flavors were added to the formula.
Methods: A mix of vitamins, minerals, and herbals was procured and mixed with excipients after screening through mesh # 20. Mixing was carried out in a V-Shell blender and finally the mix was compressed. Hardness of tablets were determined by keeping the tablets horizontally between the platens and the force required to break the tablets was recorded and later evaluated for case hardening. To ensure the uniformity of vitamins and minerals in the finished product, stratified sampling was performed during the compression of scale-up batch. Vit C and zinc were tested to ensure the uniformity of active components in the mix. Vit A constitutes 0.02% in the finished product, and therefore samples collected at start, middle, and end of compression were tested to ensure the uniformity. ASAPprime® study was performed to predict the overages used for vitamins in the formula and to get the information on final package required to maintain the shelf of finished product. ASAPprime® study consists of 21-day protocol, in which the samples are exposed to extreme temperature and humidity conditions and the associated software utilizes the Monte Carlo simulations to predict shelf life.
Results: The compressed tablet consisted of a mix of vitamins, minerals, and herbal component faced challenges during the compression. Black spots were observed on the tablets during the compression and a remedial measure was taken by using (n-1) strategy in which each component of formula was removed at a time to ascertain the causative excipient responsible for black spots. It was found that the magnesium oxide added to the formula to provide magnesium was responsible for black spots and later replaced with magnesium sulfate. We also found that the tablets undergo case hardening and gained hardness post-compression by 2-4 SCU. The target hardness set was 17.5 SCU and was comparable to reference product. Flavor concentration was evaluated and found that the concentration of 3.346% was optimal in the final formula. To ensure uniformity of actives in finished product, a total of 30 samples were collected during the run and tested for Vit C and Zinc and our results confirmed the uniformity of vitamins and minerals in the finished product. Compressed tablets were also tested for Vit A and our results showed uniform distribution of Vit A in the finished product. The results of ASAPprime® study showed that Vit A and Vit C undergo extensive degradation. Table 1 below for Vit A showed the predicted overage of 100% and one desiccant in the packaging container required to pass 3 months accelerated condition with a probability of 99%. The accelerated condition data generated for Vit A (data not shown) were in agreement with the predictions of ASAPprime® study. However, for Vit C, table 2 below showed the predicted overage of 60% and 2 desiccants in the final container required to pass 3 months accelerated condition with a probability of 97%. In contrast, our results for Vit C at accelerated conditions passed the 3 months using an overage of 35% and one desiccant. The ongoing stability results for Vit C in room temperature conditions (data not shown) is similar to the results obtained at accelerated conditions.
Conclusion: Our studies conclude that hardness of developed multivitamin chewable tablets was comparable to reference product. Among the vitamins, Vit D and Vit E did not undergo much degradation in the ASAPprime® study and accelerated temperature and humidity conditions. Predicted data by ASAPprime® study was correlated well with accelerated data of Vit A but not for Vit C, and thus warrants conducting the stability studies in accelerated and room temperature conditions.



