Back
Purpose: Periodontitis is one of the most prevalent chronic inflammatory diseases1. Recent studies identified macrophage as a promising target for periodontitis treatment because of its critical role in mediating host immune response and controlling bone absorption/regeneration2. Insulin-sensitizing peroxisomal proliferator activating receptor gamma (PPARγ) agonists showed potential to mitigate the activation of inflammatory macrophages and effectively treat periodontitis. In addition, excessive reactive oxygen species (ROS) is associated with the damage to both soft and hard tissues during the progression of periodontitis34. Therefore, ROS is another attractive target for developing novel therapeutics for periodontitis. We found that local delivery of AU9 with a novel bioactive ROS-scavenging hydrogel inhibited the activation of inflammatory macrophages, reduce the inflammatory responses, remove excessive ROS, and effectively treat periodontitis(FIG1).
Methods: We prepared a hydrogel by mixing polyvinyl alcohol (PVA) solution with the N1-(4-boronobenzyl)-N3-(4-boronophenyl)- N1, N1, N3, N3-tetramethylpropane-1, 3-diaminium (TPA) linker solution. TPGS-AU9 micelle was hydrated with TPA solution. The phenylboronic acid groups in TPA quickly react with the alcohol hydroxyl groups of PVA and form AU9 PVA-TPA hydrogels immediately after mixing.For the evaluation of AU9 and gel on cellular level. We treat macrophage cells will be RAW 264.7 murine macrophage cells treated with LPS (100 ng/mL) and AU9. Then, Dichlorofluorescein (DCF) was used as an intracellular ROS probe. We determine the ROS and the macrophage phenotype with flow cytometry, the release of pro-inflammatory cytokines with ELISA and inflammatory biomarker mRNAs with real-time qPCR respectively. For in vivo, we established a periodontitis model in C57BL/6 mice with a ligature method and real-time qPCR was used to determine the gene levels.
Results: The phenylboronic acid groups in TPA quickly react with the alcohol hydroxyl groups of PVA and form PVA-TPA hydrogels immediately after mixing (Figure 2A&B). The in vitro drug release studies showed that the hydrogel formulation released 25% in 24 hours(Figure 2C). PVA-TPA hydrogel is a ROS-scavenging bioactive material. The ability of PVA-TPA hydrogel in scavenging intracellular ROS in H2O2 treated RAW 264.7 macrophage cells were determined with fluorescence microscope (Figure 2D) and flow cytometry (Figure 2E). Dichlorofluorescein (DCF) was used as an intracellular ROS probe, which shows green fluorescence at the elevated intracellular ROS levels. Our studies also showed that PVA-TPA hydrogel effectively reduced the intracellular ROS levels in LPS-stimulated macrophage cells as determined with flow cytometry (Figure 2F). LPS could activate the NF-KB, producing inflammatory responses. Ppar-γ agonist AU9 inhibit NF-kβ (Fig3B) and show anti-inflammatory response (Fig3C).In addition, NF- kβ activation also result in the differentiation of osteoclast, which indicated by Tartrate-resistant acid phosphatase (TRAP) . RANKL is significant in stimulating osteoclast formation and activity. OPG acts as a decoy receptor for RANKL in the RANK/RANKL/OPG axis, thus down-regulating osteoclast genesis and bone resorption. The high rankl and low OPG level indicate the imbalance of bone homeostasis and ultimate tooth loss in periodontal tissue. The treatment of AU9 and hydrogel significantly decreased the trap expression. The downregulation of RANKL and upregulation of opg also exhibited the effects of AU9 and hydrogel in preventing tooth loss (Fig3F-H).
Conclusion: in this study, we found that AU9 hydrogel alleviated periodontal inflammation through modulating macrophage phenotype and scavenging of ROS.In addition, it reduced the tarp and rankl, thus preventing alveolar bone loss in experimental mice. By modulating macrophage phenotype and bone loss, this study would offer a promising therapy for common periodontitis.
References: 1. Genco, R.J. & Sanz, M. Clinical and public health implications of periodontal and systemic diseases: An overview. Periodontology 2000 83, 7-13 (2020).
2. Sima, C., Viniegra, A. & Glogauer, M. Macrophage immunomodulation in chronic osteolytic diseases-the case of periodontitis. Journal of leukocyte biology 105, 473-487 (2019).
3. Sui, L. et al. ROS-Scavenging Nanomaterials to Treat Periodontitis. Frontiers in chemistry 8, 595530-595530 (2020).
4. Zhao, H. et al. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials 258, 120286 (2020)
Acknowledgements: This work was financially supported by the following resources: Launch Innovation Award (F, Li), Auburn University start-up fund (F. Li), NIH MIRA R35GM133795 (P. Chen).
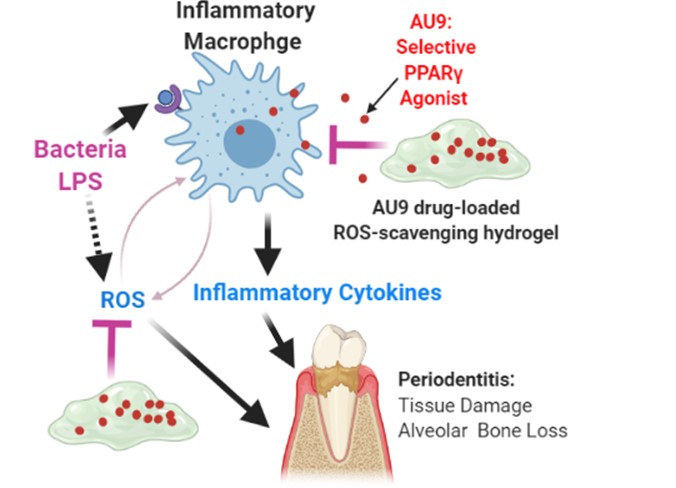
AU9 drug-loaded ROS-scavenging hydrogel for periodontitis treatment: AU9 inhibits inflammatory macrophage activation and hydrogel scavenges ROS
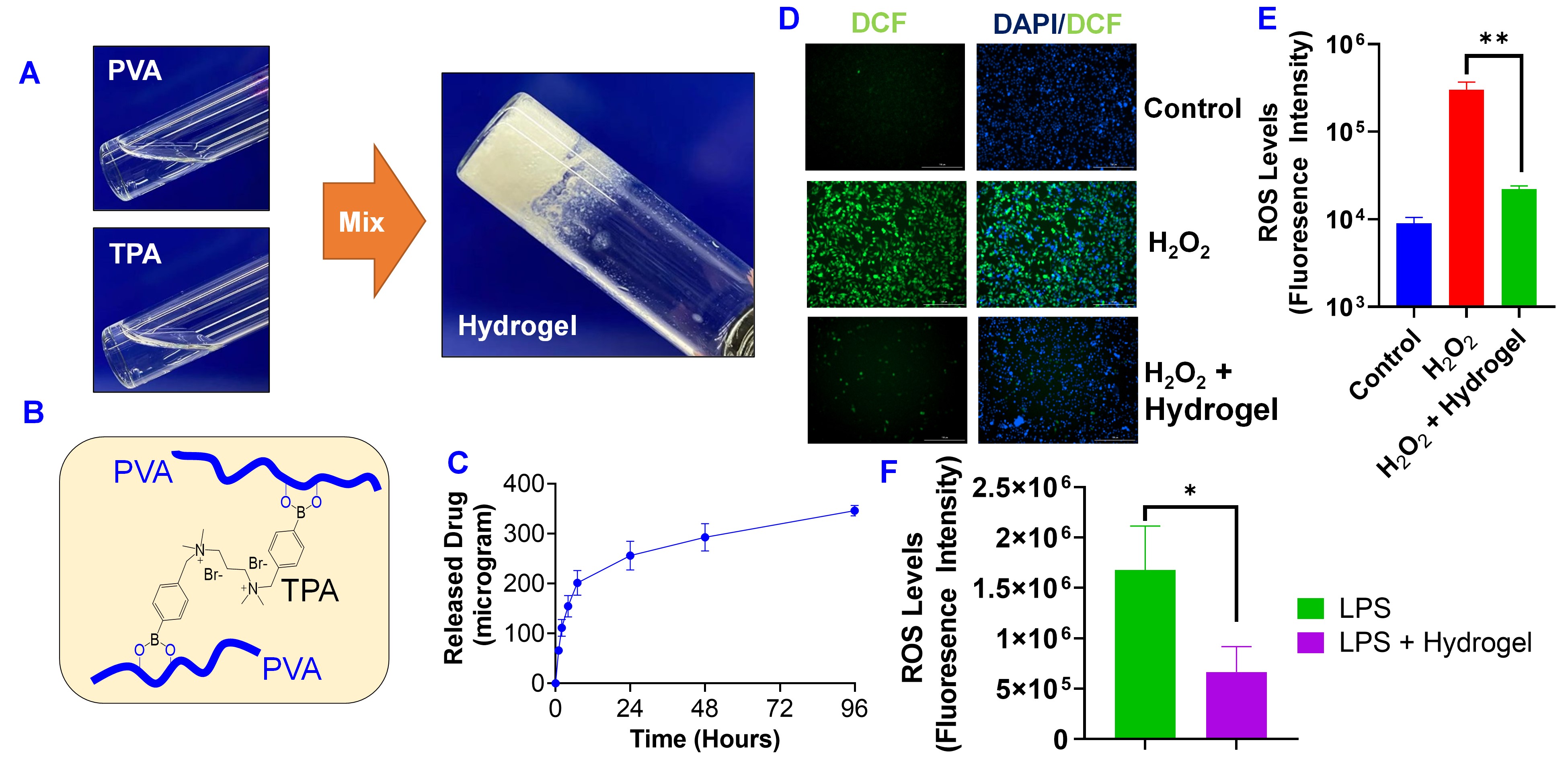
PVA-TPA hydrogel as a delivery system of AU9 with ROS scavenging ability.. (A) Preparation of hydrogel by mixing PVA and TPA solutions. (B) Chemical structure of PVA-TPA hydrogel. (C) Cumulative release of AU9 from PVA-TPA hydrogel. AU9 concentration was determined with the HPLC. PVA-TPA hydrogel scavenge intracellular ROS in macrophage cells (RAW 264.7) treated with H2O2 (0.1 mM). Intracellular ROS was determined with (D) fluorescence microscope and (E) flow cytometry. DCF was used as an intracellular ROS probe, which shows green fluorescence at the elevated intracellular ROS levels. (F) PVA-TPA hydrogel reduced intracellular ROS in RAW 264.7 cells treated with LPS as determined with flow cytometry using DCF as a ROS probe. Data are presented as the mean ± SD, n=3. *, P < 0.05; **, P < 0.01
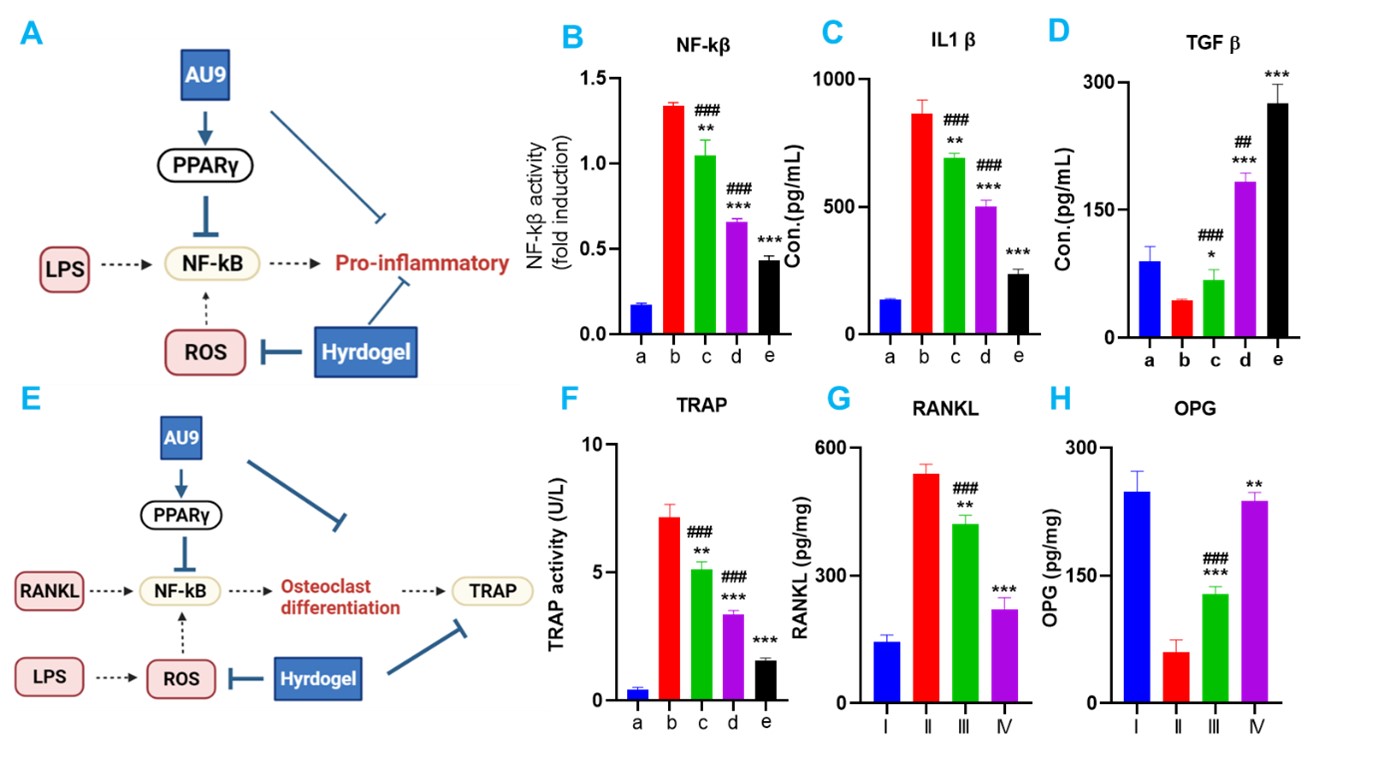
AU9 hydrogel inhibiting pro-inflammatory response and osteoclast differentiation. A. scheme of AU9 inhibiting Lps induced NF-kβ pro-inflammatory response. B NF-kβ activity in Raw264.7.C. IL-1β and D TGF-β expression in LPS-induced Raw264.7.E scheme of AU9 inhibiting osteoclast differentiation. F Tartrate-resistant acid phosphatase (TRAP) TRAP activity in monocyte. G.RANKL activity decrease with AU9 gel treatment. H OPG level increase with AU9 gel treatment.a.Control;b. LPS; c. LPS +Hydrogel; d. LPS +AU9;e. LPS +Combo. In vivo: I: control. II ligature, III ligature + Hydrogel,IV Ligature +AU9 hydrogel;
Formulation and Delivery - Biomolecular - Drug Delivery
Category: Poster Abstract
(M1530-12-68) Novel Selective PPARγ Agonist Locally Delivered with a Bioactive ROS-Scavenging Hydrogel for Periodontitis Treatment
Monday, October 17, 2022
3:30 PM – 4:30 PM ET
- XK
Xuejia Kang, Ph.D.
Auburn University
Auburn, Alabama, United States - XK
Xuejia Kang, Ph.D.
Auburn University
Auburn, Alabama, United States
Presenting Author(s)
Main Author(s)
Purpose: Periodontitis is one of the most prevalent chronic inflammatory diseases1. Recent studies identified macrophage as a promising target for periodontitis treatment because of its critical role in mediating host immune response and controlling bone absorption/regeneration2. Insulin-sensitizing peroxisomal proliferator activating receptor gamma (PPARγ) agonists showed potential to mitigate the activation of inflammatory macrophages and effectively treat periodontitis. In addition, excessive reactive oxygen species (ROS) is associated with the damage to both soft and hard tissues during the progression of periodontitis34. Therefore, ROS is another attractive target for developing novel therapeutics for periodontitis. We found that local delivery of AU9 with a novel bioactive ROS-scavenging hydrogel inhibited the activation of inflammatory macrophages, reduce the inflammatory responses, remove excessive ROS, and effectively treat periodontitis(FIG1).
Methods: We prepared a hydrogel by mixing polyvinyl alcohol (PVA) solution with the N1-(4-boronobenzyl)-N3-(4-boronophenyl)- N1, N1, N3, N3-tetramethylpropane-1, 3-diaminium (TPA) linker solution. TPGS-AU9 micelle was hydrated with TPA solution. The phenylboronic acid groups in TPA quickly react with the alcohol hydroxyl groups of PVA and form AU9 PVA-TPA hydrogels immediately after mixing.For the evaluation of AU9 and gel on cellular level. We treat macrophage cells will be RAW 264.7 murine macrophage cells treated with LPS (100 ng/mL) and AU9. Then, Dichlorofluorescein (DCF) was used as an intracellular ROS probe. We determine the ROS and the macrophage phenotype with flow cytometry, the release of pro-inflammatory cytokines with ELISA and inflammatory biomarker mRNAs with real-time qPCR respectively. For in vivo, we established a periodontitis model in C57BL/6 mice with a ligature method and real-time qPCR was used to determine the gene levels.
Results: The phenylboronic acid groups in TPA quickly react with the alcohol hydroxyl groups of PVA and form PVA-TPA hydrogels immediately after mixing (Figure 2A&B). The in vitro drug release studies showed that the hydrogel formulation released 25% in 24 hours(Figure 2C). PVA-TPA hydrogel is a ROS-scavenging bioactive material. The ability of PVA-TPA hydrogel in scavenging intracellular ROS in H2O2 treated RAW 264.7 macrophage cells were determined with fluorescence microscope (Figure 2D) and flow cytometry (Figure 2E). Dichlorofluorescein (DCF) was used as an intracellular ROS probe, which shows green fluorescence at the elevated intracellular ROS levels. Our studies also showed that PVA-TPA hydrogel effectively reduced the intracellular ROS levels in LPS-stimulated macrophage cells as determined with flow cytometry (Figure 2F). LPS could activate the NF-KB, producing inflammatory responses. Ppar-γ agonist AU9 inhibit NF-kβ (Fig3B) and show anti-inflammatory response (Fig3C).In addition, NF- kβ activation also result in the differentiation of osteoclast, which indicated by Tartrate-resistant acid phosphatase (TRAP) . RANKL is significant in stimulating osteoclast formation and activity. OPG acts as a decoy receptor for RANKL in the RANK/RANKL/OPG axis, thus down-regulating osteoclast genesis and bone resorption. The high rankl and low OPG level indicate the imbalance of bone homeostasis and ultimate tooth loss in periodontal tissue. The treatment of AU9 and hydrogel significantly decreased the trap expression. The downregulation of RANKL and upregulation of opg also exhibited the effects of AU9 and hydrogel in preventing tooth loss (Fig3F-H).
Conclusion: in this study, we found that AU9 hydrogel alleviated periodontal inflammation through modulating macrophage phenotype and scavenging of ROS.In addition, it reduced the tarp and rankl, thus preventing alveolar bone loss in experimental mice. By modulating macrophage phenotype and bone loss, this study would offer a promising therapy for common periodontitis.
References: 1. Genco, R.J. & Sanz, M. Clinical and public health implications of periodontal and systemic diseases: An overview. Periodontology 2000 83, 7-13 (2020).
2. Sima, C., Viniegra, A. & Glogauer, M. Macrophage immunomodulation in chronic osteolytic diseases-the case of periodontitis. Journal of leukocyte biology 105, 473-487 (2019).
3. Sui, L. et al. ROS-Scavenging Nanomaterials to Treat Periodontitis. Frontiers in chemistry 8, 595530-595530 (2020).
4. Zhao, H. et al. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials 258, 120286 (2020)
Acknowledgements: This work was financially supported by the following resources: Launch Innovation Award (F, Li), Auburn University start-up fund (F. Li), NIH MIRA R35GM133795 (P. Chen).

AU9 drug-loaded ROS-scavenging hydrogel for periodontitis treatment: AU9 inhibits inflammatory macrophage activation and hydrogel scavenges ROS

PVA-TPA hydrogel as a delivery system of AU9 with ROS scavenging ability.. (A) Preparation of hydrogel by mixing PVA and TPA solutions. (B) Chemical structure of PVA-TPA hydrogel. (C) Cumulative release of AU9 from PVA-TPA hydrogel. AU9 concentration was determined with the HPLC. PVA-TPA hydrogel scavenge intracellular ROS in macrophage cells (RAW 264.7) treated with H2O2 (0.1 mM). Intracellular ROS was determined with (D) fluorescence microscope and (E) flow cytometry. DCF was used as an intracellular ROS probe, which shows green fluorescence at the elevated intracellular ROS levels. (F) PVA-TPA hydrogel reduced intracellular ROS in RAW 264.7 cells treated with LPS as determined with flow cytometry using DCF as a ROS probe. Data are presented as the mean ± SD, n=3. *, P < 0.05; **, P < 0.01

AU9 hydrogel inhibiting pro-inflammatory response and osteoclast differentiation. A. scheme of AU9 inhibiting Lps induced NF-kβ pro-inflammatory response. B NF-kβ activity in Raw264.7.C. IL-1β and D TGF-β expression in LPS-induced Raw264.7.E scheme of AU9 inhibiting osteoclast differentiation. F Tartrate-resistant acid phosphatase (TRAP) TRAP activity in monocyte. G.RANKL activity decrease with AU9 gel treatment. H OPG level increase with AU9 gel treatment.a.Control;b. LPS; c. LPS +Hydrogel; d. LPS +AU9;e. LPS +Combo. In vivo: I: control. II ligature, III ligature + Hydrogel,IV Ligature +AU9 hydrogel;
