Back
Purpose: Long acting injectables, especially in situ forming implants, have attracted increasing attention for delivering proteins, peptides and new therapeutic modalities. We developed a non-invasive in vivo imaging approach to obtain improved understanding of implant formation under in vitro and in vivo conditions and its impact on drug release.
Methods: An X-ray computed tomography (CT) contrast agent, Iohexol, was used for in vivo imaging to observe implant formation. Two situ forming implant formulations, one with only iohexol, one with both iohexol and leuprolide acetate, were examined. The formulations were prepared by mixing iohexol only, or both iohexol and leuprolide acetate, with poly(lactic-co-glycolic acid) copolymer (50:50, acid endcap, 25-35 kDa) N-methyl-2-pyrrolidone (NMP) solution. In vitroformed implants were prepared by injecting 250 µL of the formulation into vials filled with 10 mL of phosphate buffer solution (PBS) at pH 7.4 and maintained in bath shaker at 37°C. The size of implants was measured for 30 days. In vitro release studies were conducted in 10 mL PBS (pH 7.4) at 37°C to obtain iohexol, leuprolide and NMP release profiles for 30 days. For in vivo formed implants, the same volume of the formulation was administered subcutaneously to rats (n=3). CT images of implants formed both in vitro and in vivo were obtained at specific time points up to one month after injection using an IVIS Spectrum CT system (PerkinElmer, USA).
Results: Based on the CT images of in vivo formed implants with only Iohexol, core-shell structures were observed, and small black cavities started to appear at early time points (Figure 1A). However, black cavities did not appear in the formulation when both Iohexol and leuprolide acetate were included in the formulation (Figure 1B). Only core-shell structures and a thin layer on the contour of the implant were observed. For in vivo formed implants with only Iohexol, morphological changes of the black cavities inside the implants were monitored. Small black cavities appeared in the shell one hour after administration and became larger until they merged together into a big cavity in the core. The size of the cavity decreased with degradation of the polymer until it disappeared. Similar to CT imaging observation of implants formed in vitro, a core-shell structure without a black cavity was observed for the formulation with both leuprolide acetate and Iohexol. From the in vitro release profiles, both Iohexol and leuprolide acetate showed bi-phasic release profiles. However, the addition of leuprolide acetate inhibited the extent of Iohexol release after the initial burst release. The size of the in vitro formed implants started to increase after being injected into the release medium and decrease after reaching a peak around day 13. Correlation of the release profiles to the size change of the implants showed that the time the implant size started to decrease was the time that Iohexol and leuprolide acetate entered the second fast-release phase which was driven by poly(lactic-co-glycolic acid) degradation.
Conclusion: Using iohexol for CT contrast assisted in real time morphological characterization of in situ-forming implants during the implant formation process. Addition of leuprolide acetate changed the release profiles of Iohexol and NMP, and inhibited the solvent exchange during burst release. This observation is supported by the CT images that black cavities form in the implants, representing the fast solvent exchange, when leuprolide acetate is not added in the formulation.
Acknowledgements: The authors would like to acknowledge the U.S. Food and Drug Administration for financial support of this research (contract number:75F40120C00136).
Disclaimer: The views expressed in this abstract do not reflect the official policies of the U.S. Food and Drug Administration or the U.S. Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.

Figure 1.A CT images of implants at various time post implantation (Iohexol only_with cavity)
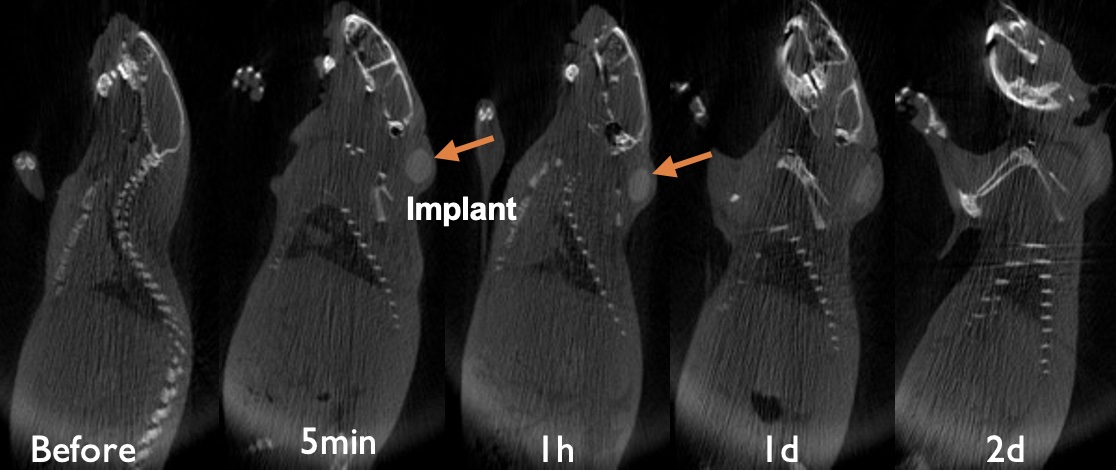
Figure 1.B CT images of implants at various time post implantation (Iohexol and leuprolide acetate_no cavity was observed)
Formulation and Delivery - Chemical - Drug Delivery
Category: Poster Abstract
(M1530-04-21) Assessing In Situ Forming Implants Using Real-Time Imaging
Monday, October 17, 2022
3:30 PM – 4:30 PM ET
- XL
Xinhao Lin, BS
University of Connecticut
Storrs, Connecticut, United States - XL
Xinhao Lin, BS
University of Connecticut
Storrs, Connecticut, United States
Presenting Author(s)
Main Author(s)
Purpose: Long acting injectables, especially in situ forming implants, have attracted increasing attention for delivering proteins, peptides and new therapeutic modalities. We developed a non-invasive in vivo imaging approach to obtain improved understanding of implant formation under in vitro and in vivo conditions and its impact on drug release.
Methods: An X-ray computed tomography (CT) contrast agent, Iohexol, was used for in vivo imaging to observe implant formation. Two situ forming implant formulations, one with only iohexol, one with both iohexol and leuprolide acetate, were examined. The formulations were prepared by mixing iohexol only, or both iohexol and leuprolide acetate, with poly(lactic-co-glycolic acid) copolymer (50:50, acid endcap, 25-35 kDa) N-methyl-2-pyrrolidone (NMP) solution. In vitroformed implants were prepared by injecting 250 µL of the formulation into vials filled with 10 mL of phosphate buffer solution (PBS) at pH 7.4 and maintained in bath shaker at 37°C. The size of implants was measured for 30 days. In vitro release studies were conducted in 10 mL PBS (pH 7.4) at 37°C to obtain iohexol, leuprolide and NMP release profiles for 30 days. For in vivo formed implants, the same volume of the formulation was administered subcutaneously to rats (n=3). CT images of implants formed both in vitro and in vivo were obtained at specific time points up to one month after injection using an IVIS Spectrum CT system (PerkinElmer, USA).
Results: Based on the CT images of in vivo formed implants with only Iohexol, core-shell structures were observed, and small black cavities started to appear at early time points (Figure 1A). However, black cavities did not appear in the formulation when both Iohexol and leuprolide acetate were included in the formulation (Figure 1B). Only core-shell structures and a thin layer on the contour of the implant were observed. For in vivo formed implants with only Iohexol, morphological changes of the black cavities inside the implants were monitored. Small black cavities appeared in the shell one hour after administration and became larger until they merged together into a big cavity in the core. The size of the cavity decreased with degradation of the polymer until it disappeared. Similar to CT imaging observation of implants formed in vitro, a core-shell structure without a black cavity was observed for the formulation with both leuprolide acetate and Iohexol. From the in vitro release profiles, both Iohexol and leuprolide acetate showed bi-phasic release profiles. However, the addition of leuprolide acetate inhibited the extent of Iohexol release after the initial burst release. The size of the in vitro formed implants started to increase after being injected into the release medium and decrease after reaching a peak around day 13. Correlation of the release profiles to the size change of the implants showed that the time the implant size started to decrease was the time that Iohexol and leuprolide acetate entered the second fast-release phase which was driven by poly(lactic-co-glycolic acid) degradation.
Conclusion: Using iohexol for CT contrast assisted in real time morphological characterization of in situ-forming implants during the implant formation process. Addition of leuprolide acetate changed the release profiles of Iohexol and NMP, and inhibited the solvent exchange during burst release. This observation is supported by the CT images that black cavities form in the implants, representing the fast solvent exchange, when leuprolide acetate is not added in the formulation.
Acknowledgements: The authors would like to acknowledge the U.S. Food and Drug Administration for financial support of this research (contract number:75F40120C00136).
Disclaimer: The views expressed in this abstract do not reflect the official policies of the U.S. Food and Drug Administration or the U.S. Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.

Figure 1.A CT images of implants at various time post implantation (Iohexol only_with cavity)

Figure 1.B CT images of implants at various time post implantation (Iohexol and leuprolide acetate_no cavity was observed)
