Back
Purpose: Pulmonary tuberculosis (TB) is caused by Mycobacterium tuberculosis (Mtb) and has the highest mortality rate among infectious diseases. The drug regimen for TB treatment is administered orally involving prolonged treatment resulting in severe adverse effects and poor patient compliance. Often poor patient compliance results in development of multi-drug resistant strains of bacteria. In recent times, pulmonary delivery system of anti-TB drugs has gained enormous interest as it provides various benefits like larger surface area, localized therapy, overcoming first pass metabolism, reduced systemic adverse effects, enhanced bioavailability, and rapid onset of action. However, delamanid (DLD), an antituberculosis drug, has poor aqueous solubility limiting its direct delivery into the lungs. We therefore developed a cyclodextrin complexation of DLD to improve the solubility. Cyclodextrins are hollow truncated cone shaped cyclic oligosaccharides with a hydrophilic outer surface and a lipophilic cavity capable of attracting and entrapping lipophilic drugs. Further, the DLD-CD complex will enable its direct administration into the lungs for better patient compliance and enhanced treatment efficacy.
Methods: Phase solubility studies of DLD-CD inclusion complex were conducted by dissolving various cyclodextrins SB-β-CD (Captisol (0 to 200mM)), HP- β-CD (Cavasol HP W7 (0 to 200mM)) and HP- ɣ-CD (Cavasol HP W8 (0 to 200mM)) in deionized water with excess amount of drug (2 mg). These samples are subjected to sonication followed by overnight stirring at room temperature. The free un-complexed DLD was separated using filtration through the 0.45µm filter and drug content was analyzed using UV spectrometry at 224 nm. For continuous variation plot study, both cyclodextrin and DLD were added to prepare one molar fraction concentration by varying concentrations from 0.0 to 1.0. These solutions were subjected to sonication and stirring overnight at room temperature. Later, solutions were filtered through 0.45µm filter and quantified using UV spectrometry. Then, DLD-CD complexes were freeze-dried (Labconco freeze dryer 4.5) and the physical nature of the DLD were assessed via solid-state characterization studies such as Differential Scanning Calorimetry (DSC) and Powder X-Ray Diffraction analysis (PXRD). The formulation was later assessed for in vitro aerosolization studies (Next Generation impactor) by nebulization to understand aerosolization performance of the DLD-CD complex. Additional MIC and stability studies were conducted in Mtb (H37Ra strain) to evaluate the efficacy and stability of CD complex.
Results: Sulfobutyl ether-b-cyclodextrin (SBE-b-CD) and Hydroxypropyl-b-cyclodextrin (HP-b-CD) were screened for solubility enhancement and it was found that HP< ![if !supportAnnotations] >[NK1]< ![endif] > < ![if !supportAnnotations] >[DB2]< ![endif] > -b-CD resulted in 54-fold increase in solubility compared to 27-fold increase by SBE-b-CD. The stability constant (Kc; 265 ± 15 M-1) and complexation efficiency (CE; 8.5 x 10-4) suggest formation of a stable inclusion complex of DLD and HP-b-CD in 2:1 ratio. The complex formation was confirmed by physical characterizations using DSC, PXRD, NMR and FTIR studies. The DSC thermograph of the DLD powder showed a single sharp endothermic peak at 189◦C (figure 2A) indicating melting point and crystalline nature of the drug. The characteristic melting endothermic peak of DLD was absent in the DSC thermogram of DLD-CD complex showing that DLD is completely included in the cavity of cyclodextrin in amorphous state. The PXRD studies (figure 2B) of DLD demonstrates intense peaks from 17 to 22°, indicating the crystalline nature of drug. However, PXRD of the DLD-CD showed broad and diffuse pattern with no intense peaks, indicating an essentially amorphous nature. Thus providing evidence that DLD was completely entrapped in cavity of HP-β-CD, this is in accordance with DSC data. The in vitro aerosolization (figure 3) of nebulized DLD-CD complex solution showed an MMAD of 4.42 ± 0.62 μm and fine particle fraction, (FPF) was 82.28 ± 2.79%, suggesting that particles are within the reported size range (1 – 5 μm) for deposition in the respiratory region of lungs with majority of the dose deposition in the bronchial-alveolar regions of the lungs. The DLD-CD complex was stable for 8 weeks in accelerated stability studies. In bacterial studies, the minimum inhibitory concentration of DLD-CD complex was significantly reduced (4-fold) as compared to free DLD in Mtb (H37Ra strain).
Conclusion: In conclusion, the aqueous solubility of the DLD was improved through cyclodextrin complexation. Moreover, the DLD-CD complex demonstrated promising pulmonary delivery potential with MMAD of 4.42 ± 0.62 μm and fine particle fraction, (FPF) of 82.28 ± 2.79%. Inclusion complex provided higher antibacterial activity against M. tuberculosis in comparison to DLD. Therefore, the DLD- HP-β-CD complex with improved solubility, enhanced antibacterial activity was successfully developed for tuberculosis treatment.
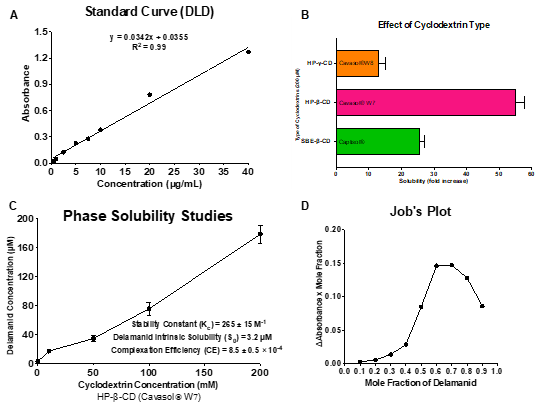
Figure 1. (A) Calibration curve for Delamanid plotted using UV Spectrophotometer from the standard solutions ranging from 0-40 µg/mL at 224 nm. (B) Effect of cyclodextrin type viz. Captisol®, Cavasol® W7 and Cavasol® W8 on aqueous solubility of DLD. (C) Phase solubility diagram of Delamanid with hydroxypropyl β-Cyclodextrin (HP-β-CD) in water. (D) Job’s continuous variation plot of Delamanid-CD complex suggesting 2:1 drug-cyclodextrin stoichiometry of inclusion complex. All data represent mean ± SD (n=3).
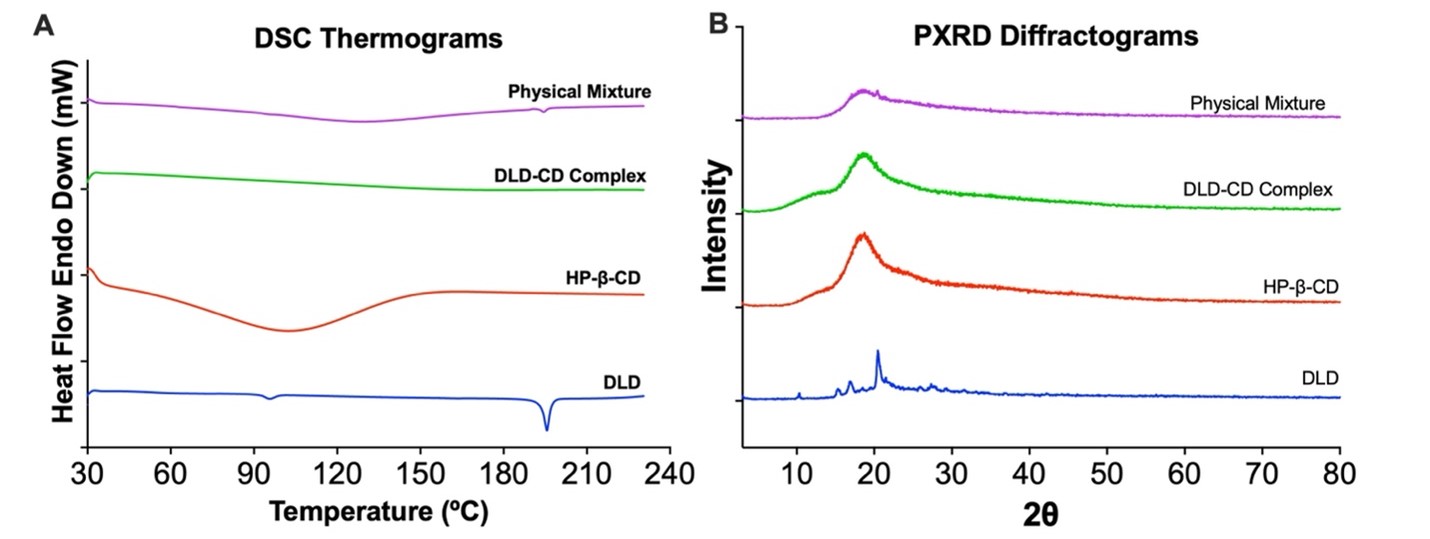
Figure 2. (A) DSC thermograms and (B) PXRD spectra of delamanid, cyclodextrin, delamanid-cyclodextrin complex and physical mixture of delamanid and HP-β-CD.
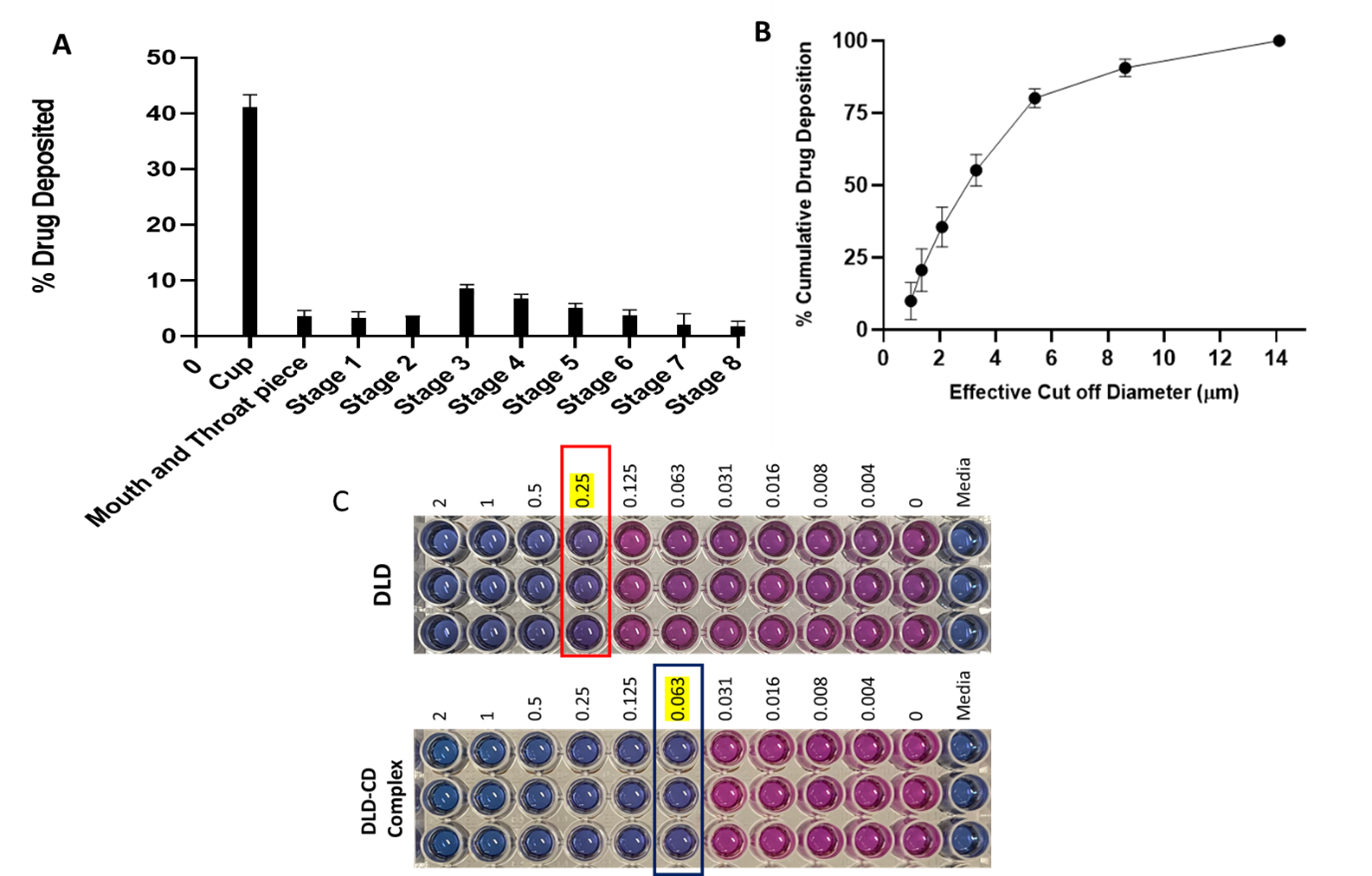
Figure 3. (A) In vitro aerosolization performance of DLD-CD and Drug deposition at various stages of NGI. (B) Cumulative drug deposition as function of cutoff diameter of NGI stages. (C) Minimum inhibitory concentration (MIC) estimation using resazurin-based microtiter plate (REMA) assay. Pink color in wells refer to growth in bacteria whereas blue color represents the bacterial growth inhibition.
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(M1530-04-19) Solubility Enhancement and Improved Efficacy of Delamanid Using Cyclodextrin Inclusion Complex
Monday, October 17, 2022
3:30 PM – 4:30 PM ET
- DB
Druva Sarika Barji, MS
St. John's University
Queens, New York, United States - NK
Nitesh K. Kunda, Ph.D.
St. John's University
Queens, New York, United States
Presenting Author(s)
Main Author(s)
Purpose: Pulmonary tuberculosis (TB) is caused by Mycobacterium tuberculosis (Mtb) and has the highest mortality rate among infectious diseases. The drug regimen for TB treatment is administered orally involving prolonged treatment resulting in severe adverse effects and poor patient compliance. Often poor patient compliance results in development of multi-drug resistant strains of bacteria. In recent times, pulmonary delivery system of anti-TB drugs has gained enormous interest as it provides various benefits like larger surface area, localized therapy, overcoming first pass metabolism, reduced systemic adverse effects, enhanced bioavailability, and rapid onset of action. However, delamanid (DLD), an antituberculosis drug, has poor aqueous solubility limiting its direct delivery into the lungs. We therefore developed a cyclodextrin complexation of DLD to improve the solubility. Cyclodextrins are hollow truncated cone shaped cyclic oligosaccharides with a hydrophilic outer surface and a lipophilic cavity capable of attracting and entrapping lipophilic drugs. Further, the DLD-CD complex will enable its direct administration into the lungs for better patient compliance and enhanced treatment efficacy.
Methods: Phase solubility studies of DLD-CD inclusion complex were conducted by dissolving various cyclodextrins SB-β-CD (Captisol (0 to 200mM)), HP- β-CD (Cavasol HP W7 (0 to 200mM)) and HP- ɣ-CD (Cavasol HP W8 (0 to 200mM)) in deionized water with excess amount of drug (2 mg). These samples are subjected to sonication followed by overnight stirring at room temperature. The free un-complexed DLD was separated using filtration through the 0.45µm filter and drug content was analyzed using UV spectrometry at 224 nm. For continuous variation plot study, both cyclodextrin and DLD were added to prepare one molar fraction concentration by varying concentrations from 0.0 to 1.0. These solutions were subjected to sonication and stirring overnight at room temperature. Later, solutions were filtered through 0.45µm filter and quantified using UV spectrometry. Then, DLD-CD complexes were freeze-dried (Labconco freeze dryer 4.5) and the physical nature of the DLD were assessed via solid-state characterization studies such as Differential Scanning Calorimetry (DSC) and Powder X-Ray Diffraction analysis (PXRD). The formulation was later assessed for in vitro aerosolization studies (Next Generation impactor) by nebulization to understand aerosolization performance of the DLD-CD complex. Additional MIC and stability studies were conducted in Mtb (H37Ra strain) to evaluate the efficacy and stability of CD complex.
Results: Sulfobutyl ether-b-cyclodextrin (SBE-b-CD) and Hydroxypropyl-b-cyclodextrin (HP-b-CD) were screened for solubility enhancement and it was found that HP< ![if !supportAnnotations] >[NK1]< ![endif] > < ![if !supportAnnotations] >[DB2]< ![endif] > -b-CD resulted in 54-fold increase in solubility compared to 27-fold increase by SBE-b-CD. The stability constant (Kc; 265 ± 15 M-1) and complexation efficiency (CE; 8.5 x 10-4) suggest formation of a stable inclusion complex of DLD and HP-b-CD in 2:1 ratio. The complex formation was confirmed by physical characterizations using DSC, PXRD, NMR and FTIR studies. The DSC thermograph of the DLD powder showed a single sharp endothermic peak at 189◦C (figure 2A) indicating melting point and crystalline nature of the drug. The characteristic melting endothermic peak of DLD was absent in the DSC thermogram of DLD-CD complex showing that DLD is completely included in the cavity of cyclodextrin in amorphous state. The PXRD studies (figure 2B) of DLD demonstrates intense peaks from 17 to 22°, indicating the crystalline nature of drug. However, PXRD of the DLD-CD showed broad and diffuse pattern with no intense peaks, indicating an essentially amorphous nature. Thus providing evidence that DLD was completely entrapped in cavity of HP-β-CD, this is in accordance with DSC data. The in vitro aerosolization (figure 3) of nebulized DLD-CD complex solution showed an MMAD of 4.42 ± 0.62 μm and fine particle fraction, (FPF) was 82.28 ± 2.79%, suggesting that particles are within the reported size range (1 – 5 μm) for deposition in the respiratory region of lungs with majority of the dose deposition in the bronchial-alveolar regions of the lungs. The DLD-CD complex was stable for 8 weeks in accelerated stability studies. In bacterial studies, the minimum inhibitory concentration of DLD-CD complex was significantly reduced (4-fold) as compared to free DLD in Mtb (H37Ra strain).
Conclusion: In conclusion, the aqueous solubility of the DLD was improved through cyclodextrin complexation. Moreover, the DLD-CD complex demonstrated promising pulmonary delivery potential with MMAD of 4.42 ± 0.62 μm and fine particle fraction, (FPF) of 82.28 ± 2.79%. Inclusion complex provided higher antibacterial activity against M. tuberculosis in comparison to DLD. Therefore, the DLD- HP-β-CD complex with improved solubility, enhanced antibacterial activity was successfully developed for tuberculosis treatment.

Figure 1. (A) Calibration curve for Delamanid plotted using UV Spectrophotometer from the standard solutions ranging from 0-40 µg/mL at 224 nm. (B) Effect of cyclodextrin type viz. Captisol®, Cavasol® W7 and Cavasol® W8 on aqueous solubility of DLD. (C) Phase solubility diagram of Delamanid with hydroxypropyl β-Cyclodextrin (HP-β-CD) in water. (D) Job’s continuous variation plot of Delamanid-CD complex suggesting 2:1 drug-cyclodextrin stoichiometry of inclusion complex. All data represent mean ± SD (n=3).

Figure 2. (A) DSC thermograms and (B) PXRD spectra of delamanid, cyclodextrin, delamanid-cyclodextrin complex and physical mixture of delamanid and HP-β-CD.

Figure 3. (A) In vitro aerosolization performance of DLD-CD and Drug deposition at various stages of NGI. (B) Cumulative drug deposition as function of cutoff diameter of NGI stages. (C) Minimum inhibitory concentration (MIC) estimation using resazurin-based microtiter plate (REMA) assay. Pink color in wells refer to growth in bacteria whereas blue color represents the bacterial growth inhibition.
