Back
Purpose: Drug colloids, such as surfactant and bile micelles, as well as crystalline and amorphous drug particles, have been shown to enhance the permeation and absorption of poorly soluble drugs. This was predominantly attributed to the particle drifting effect, where colloidal drug particles move into the unstirred water layer (UWL) by acting as shuttles and releasing the drug near the membrane surface, thereby facilitating passive permeation. The extent of particle drifting effect can be calculated using Fick’s law of diffusion, where permeation enhancement is dependent on the diffusion coefficient and concentration of the colloid of interest, as well as the thickness of the UWL. Previously, the thickness of the UWL was assumed to be constant for the free drug, micelles, and particles, as well as particles of different sizes. This study examines how different diffusing species affect UWL thickness, thus leading to altered drug mass transport rate across the UWL.
Methods: A biphasic diffusion setup (Figure 1A) consisting of two phases (50 mL pH 6.8 phosphate buffer and 50 mL decanol) was used to determine the thickness of UWL at the water-decanol interface, which provides major diffusional resistance for drug to transfer to the organic phase. This system was maintained at 37°C and agitated by an overhead stirrer with one shaft that contains two impellers for each layer of solution to minimize diffusional resistance of decanol phase. The stirring rate was set at 90 rpm to ensure homogeneous hydrodynamic mixing in both media while preserving a stable aqueous-decanol interphase. After adding a pre-determined amount of drug predissolved in an organic solvent (addition kept below 2%) into the aqueous buffer, the concentration of the drug transferred to the decanol phase was monitored using a fiber optics UV dip probe (SI Photonics, Tuscon, AZ) as a function of time. The decay constant (Kp), which reflects the drug partitioning rate into the decanol phase, was calculated using a mechanistic mass transport model1 (Figure 1B) using MATLAB (MathWorks, Natick, MA). The diffusion coefficient of the drug colloid of interest was measured using dynamic light scattering (DLS). These values were then used to calculate unstirred water layer (UWL) thickness (hUWL) (Figure 1B). Atazanavir was used as a model drug given its high UV absorbance and ease of particle size control.
Results: Atazanavir amorphous particles of various sizes (150, 200, 300, 400, and 600 nm) were generated using varying stirring rates and stock solutions with different drug concentrations using HPMCAS as a stabilizer. The size of particles before and after diffusion experiments was confirmed to remain unchanged using DLS. The UWL thicknesses of particles for different sizes are summarized in Figure 2. A linear relationship between hUWL and the one-third power of the diffusion coefficient (D) was obtained, with bigger particles having thinner UWLs and smaller particles having thicker UWLs. Similarly, thicker UWLs were obtained with the free drug compared to amorphous particles and bile salt micelles (Figure 1B). These results confirmed the particle drifting effect hypothesis brought forth by Sugano et al.2, where the presence of drug particles leads to a reduction in UWL thickness compared to the free drug alone. Also, although smaller particles diffuse faster into the UWL than larger particles, the extent of particle drifting effect may be mitigated to a certain extent due to an increase in UWL thickness by smaller particles. Furthermore, these results suggested that it would be unreasonable to use a unified UWL thickness in the prediction of particle drifting effect, as the size of the UWL varies with the size of species present in the UWL.
Using atazanavir amorphous particles of 300 nm diameter as a model system, the impact of corona type on UWL thickness was tested. Various commonly used pharmaceutical excipients was evaluated at 100 µg/mL with particle concentrations ranging from 100 to 150 µg/mL, and results are summarized in Figure 3. The amorphous solubility and particle size for atazanavir remained nearly unchanged. It can be seen that UWL thickness remained constant as long as particle size (diffusion coefficient) remained the same, regardless of the type of excipient used. These results suggested that the particle drifting effect across the UWL is independent of particle corona type. Therefore, particle size is the main factor that affects the extent of particle drifting effect through altering both the diffusion rate in UWL and the thickness of the UWL.
Conclusion: The thickness of the UWL was found to be dependent on the type of diffusing species and particle size. Thicker UWLs were observed for species with larger diffusion coefficients, and vice versa. Excipient corona type exhibited minimal impact on UWL thickness. Therefore, to better predict the particle drifting effect, accurate determination of UWL thickness for the species of interest appears to be necessary. Results from this study should aid in improving the prediction of particle drifting effect and bioavailability enhancement for colloid-containing formulations.
References: 1. Mudie, D. M.; Shi, Y.; Ping, H.; Gao, P.; Amidon, G. L.; Amidon, G. E. Mechanistic analysis of solute transport in an in vitro physiological two-phase dissolution apparatus. Biopharmaceutics & drug disposition 2012, 33, (7), 378-402.
2. Sugano, K. Possible reduction of effective thickness of intestinal unstirred water layer by particle drifting effect. Int. J. Pharm. 2010, 387, (1), 103-109.
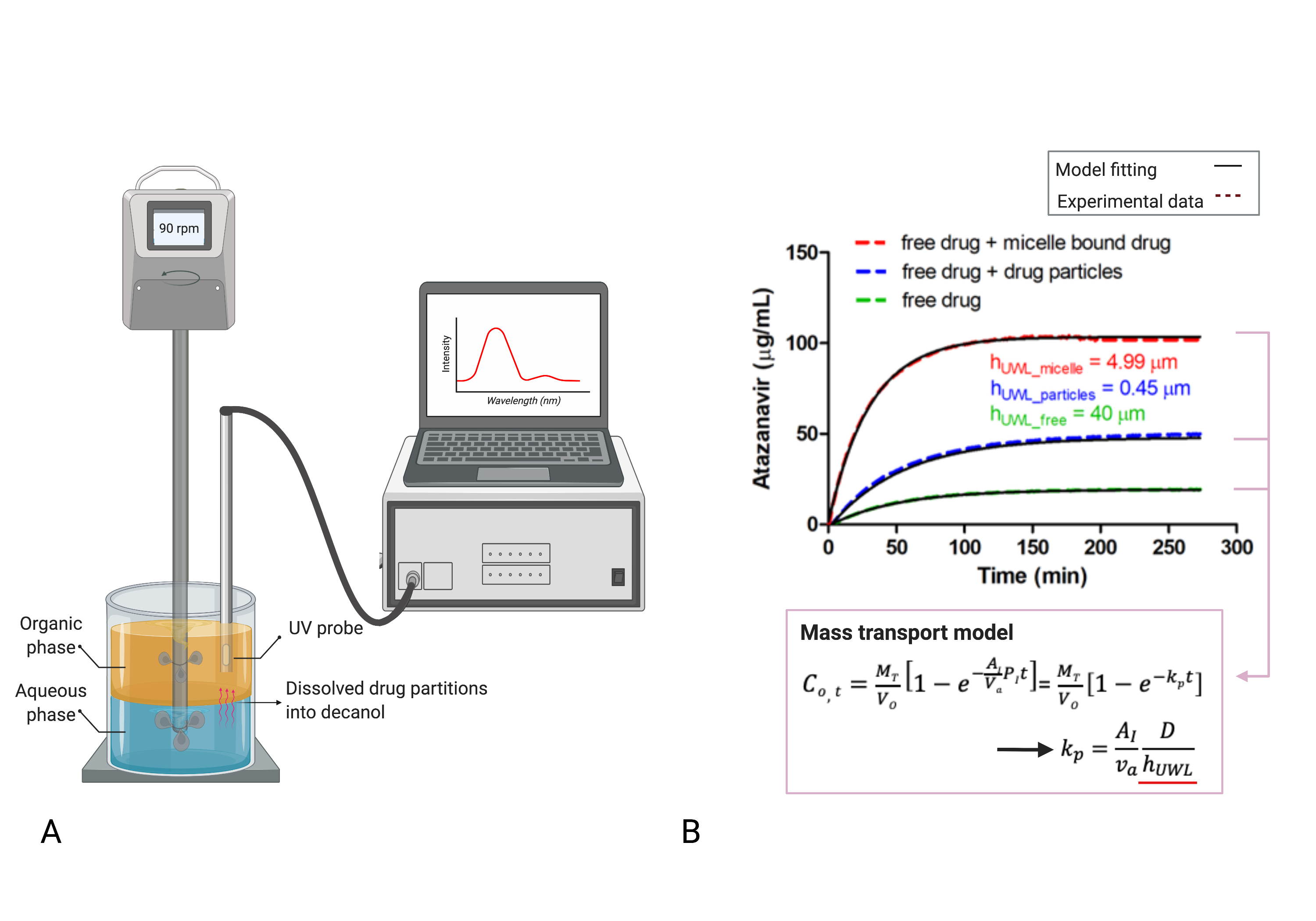
Figure 1. A) Schematic of the biphasic dissolution experimental setup, and B) mass transport model used to calculate UWL thickness
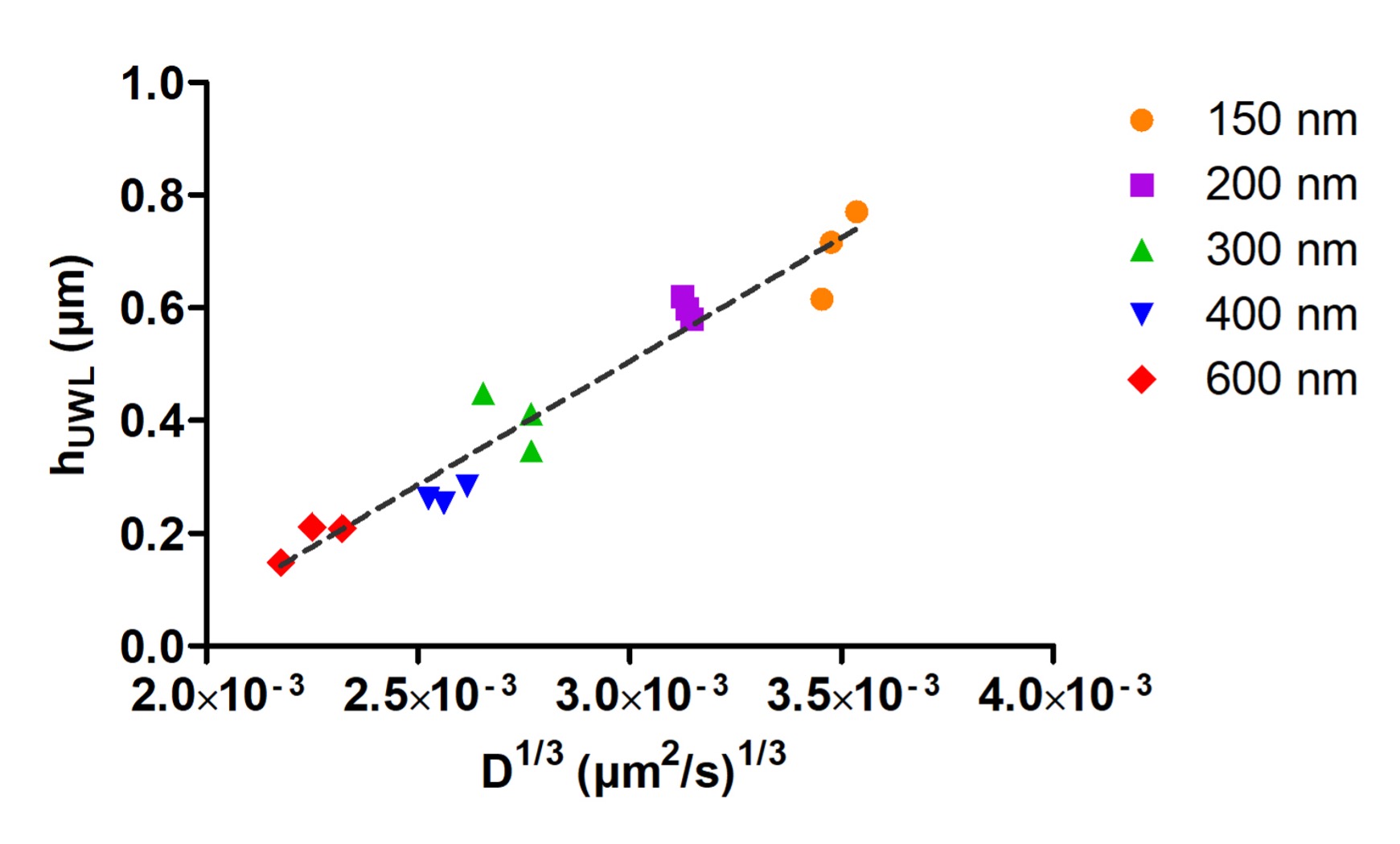
Figure 2. Linear relationship between UWL thickness and one-third power of diffusion coefficient for atazanavir amorphous particles.
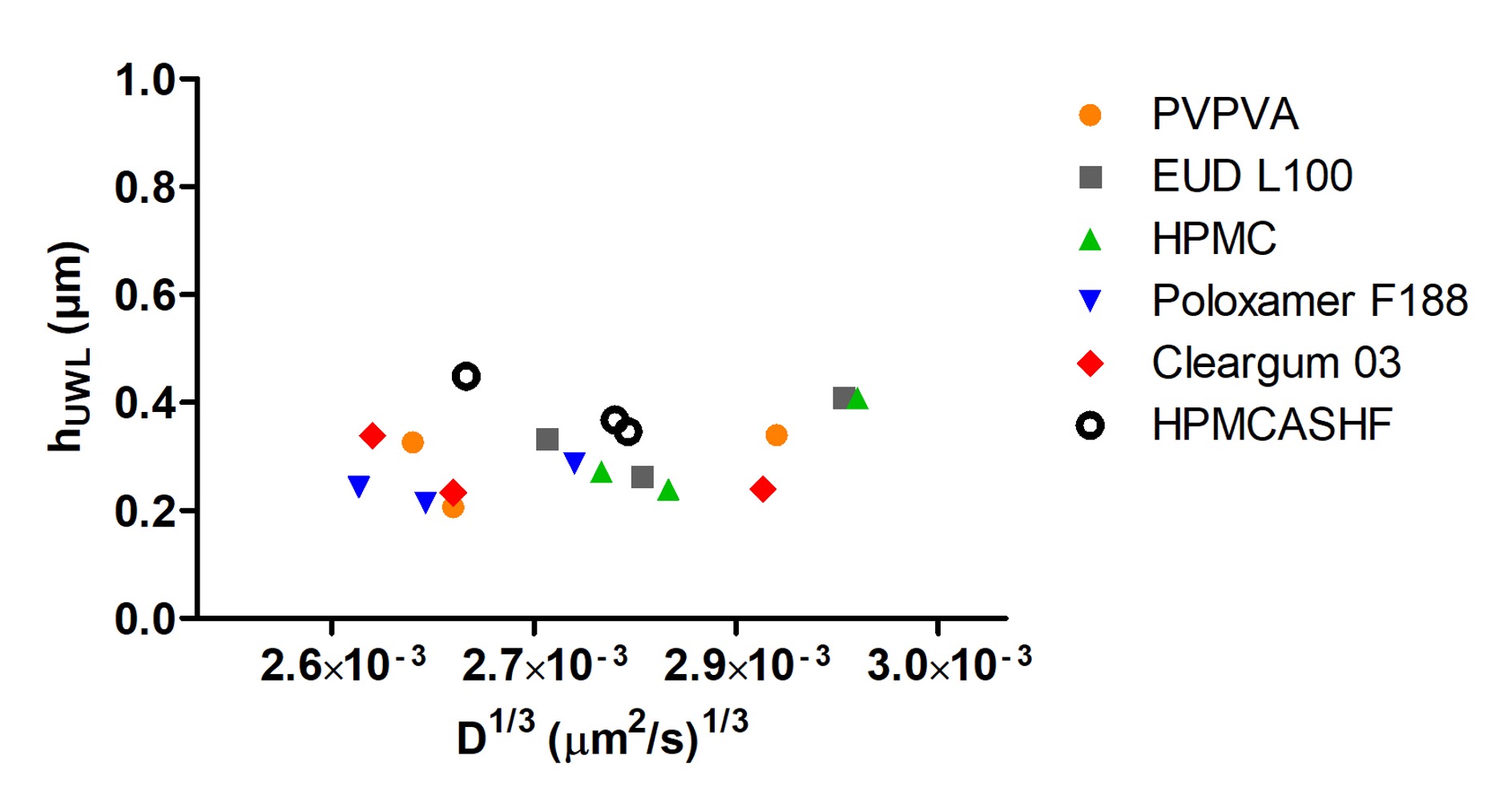
Figure 3. Impact of corona type on UWL thickness for atazanavir amorphous particles
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(M1430-07-41) The Size of the Unstirred Water Layer as a Function of Diffusion Coefficient
Monday, October 17, 2022
2:30 PM – 3:30 PM ET
- DY
Da Hye Yang, MS
University of Connecticut
Storrs, Connecticut, United States - DY
Da Hye Yang, MS
University of Connecticut
Storrs, Connecticut, United States
Presenting Author(s)
Main Author(s)
Purpose: Drug colloids, such as surfactant and bile micelles, as well as crystalline and amorphous drug particles, have been shown to enhance the permeation and absorption of poorly soluble drugs. This was predominantly attributed to the particle drifting effect, where colloidal drug particles move into the unstirred water layer (UWL) by acting as shuttles and releasing the drug near the membrane surface, thereby facilitating passive permeation. The extent of particle drifting effect can be calculated using Fick’s law of diffusion, where permeation enhancement is dependent on the diffusion coefficient and concentration of the colloid of interest, as well as the thickness of the UWL. Previously, the thickness of the UWL was assumed to be constant for the free drug, micelles, and particles, as well as particles of different sizes. This study examines how different diffusing species affect UWL thickness, thus leading to altered drug mass transport rate across the UWL.
Methods: A biphasic diffusion setup (Figure 1A) consisting of two phases (50 mL pH 6.8 phosphate buffer and 50 mL decanol) was used to determine the thickness of UWL at the water-decanol interface, which provides major diffusional resistance for drug to transfer to the organic phase. This system was maintained at 37°C and agitated by an overhead stirrer with one shaft that contains two impellers for each layer of solution to minimize diffusional resistance of decanol phase. The stirring rate was set at 90 rpm to ensure homogeneous hydrodynamic mixing in both media while preserving a stable aqueous-decanol interphase. After adding a pre-determined amount of drug predissolved in an organic solvent (addition kept below 2%) into the aqueous buffer, the concentration of the drug transferred to the decanol phase was monitored using a fiber optics UV dip probe (SI Photonics, Tuscon, AZ) as a function of time. The decay constant (Kp), which reflects the drug partitioning rate into the decanol phase, was calculated using a mechanistic mass transport model1 (Figure 1B) using MATLAB (MathWorks, Natick, MA). The diffusion coefficient of the drug colloid of interest was measured using dynamic light scattering (DLS). These values were then used to calculate unstirred water layer (UWL) thickness (hUWL) (Figure 1B). Atazanavir was used as a model drug given its high UV absorbance and ease of particle size control.
Results: Atazanavir amorphous particles of various sizes (150, 200, 300, 400, and 600 nm) were generated using varying stirring rates and stock solutions with different drug concentrations using HPMCAS as a stabilizer. The size of particles before and after diffusion experiments was confirmed to remain unchanged using DLS. The UWL thicknesses of particles for different sizes are summarized in Figure 2. A linear relationship between hUWL and the one-third power of the diffusion coefficient (D) was obtained, with bigger particles having thinner UWLs and smaller particles having thicker UWLs. Similarly, thicker UWLs were obtained with the free drug compared to amorphous particles and bile salt micelles (Figure 1B). These results confirmed the particle drifting effect hypothesis brought forth by Sugano et al.2, where the presence of drug particles leads to a reduction in UWL thickness compared to the free drug alone. Also, although smaller particles diffuse faster into the UWL than larger particles, the extent of particle drifting effect may be mitigated to a certain extent due to an increase in UWL thickness by smaller particles. Furthermore, these results suggested that it would be unreasonable to use a unified UWL thickness in the prediction of particle drifting effect, as the size of the UWL varies with the size of species present in the UWL.
Using atazanavir amorphous particles of 300 nm diameter as a model system, the impact of corona type on UWL thickness was tested. Various commonly used pharmaceutical excipients was evaluated at 100 µg/mL with particle concentrations ranging from 100 to 150 µg/mL, and results are summarized in Figure 3. The amorphous solubility and particle size for atazanavir remained nearly unchanged. It can be seen that UWL thickness remained constant as long as particle size (diffusion coefficient) remained the same, regardless of the type of excipient used. These results suggested that the particle drifting effect across the UWL is independent of particle corona type. Therefore, particle size is the main factor that affects the extent of particle drifting effect through altering both the diffusion rate in UWL and the thickness of the UWL.
Conclusion: The thickness of the UWL was found to be dependent on the type of diffusing species and particle size. Thicker UWLs were observed for species with larger diffusion coefficients, and vice versa. Excipient corona type exhibited minimal impact on UWL thickness. Therefore, to better predict the particle drifting effect, accurate determination of UWL thickness for the species of interest appears to be necessary. Results from this study should aid in improving the prediction of particle drifting effect and bioavailability enhancement for colloid-containing formulations.
References: 1. Mudie, D. M.; Shi, Y.; Ping, H.; Gao, P.; Amidon, G. L.; Amidon, G. E. Mechanistic analysis of solute transport in an in vitro physiological two-phase dissolution apparatus. Biopharmaceutics & drug disposition 2012, 33, (7), 378-402.
2. Sugano, K. Possible reduction of effective thickness of intestinal unstirred water layer by particle drifting effect. Int. J. Pharm. 2010, 387, (1), 103-109.

Figure 1. A) Schematic of the biphasic dissolution experimental setup, and B) mass transport model used to calculate UWL thickness

Figure 2. Linear relationship between UWL thickness and one-third power of diffusion coefficient for atazanavir amorphous particles.

Figure 3. Impact of corona type on UWL thickness for atazanavir amorphous particles
