Back
Purpose: In hydrophilic polymeric matrix systems, drug is homogeneously dispersed in a rate-controlling polymer. There are several factors that affect drug release from these matrices, and knowledge about these factors and their influence is crucial for development of optimal formulation. When these matrices are placed in a dissolution medium, polymer hydrates and forms a gel diffusion layer which controls the release kinetics. Factors that influence formation and thickness of the diffusion layer determine rate of drug release. The type of polymer and its concentration determine the rate of water uptake, erosion of polymer, and thus formation of diffusional gel layer. The compression pressure is a critical process parameter affecting drug release. It governs number of pores matrix has for eluting fluid to penetrate and interact with polymer to form gel layer around matrix. Changing the geometry of the matrix tablets changes the surface area available for interaction with dissolution medium. In this study, an attempt was made to understand the factors, i.e. tablet geometry, the ratio of rate-controlling polymer in the matrix, and compression pressure, which influence the drug release from matrix system and optimize them using a central composite design.
Methods: In present study, a central composite design was used to study the effect of independent factors on dependent factors (responses). The experimental design consisted of three independent factors, viz. non-ionic:ionic polymer ratio (X1), compression pressure (X2), and tablet geometry (X3). These factors were studied at three levels, i.e. low, medium and high (-1, 0, +1). Tablet geometry was considered a categorical factor. Matrix tablets of cylindrical, round and capsule shapes were used in the study. The levels of independent factors for experimental designs were determined from initial screening experiments. Percent swelling, percent erosion, gel diffusion layer thickness, and drug release after 1, 6 and 12 hours were selected as dependent factors. Verapamil hydrochloride was selected as a model drug based on its high solubility in water. Tablets were prepared by direct compression of drug and polymer mixture in a 1:3 ratio. HPMC K4M and Eudragit® RSPO were selected as matrix-forming polymers. In order to record responses, dissolution, water uptake and erosion studies were conducted in dissolution media representing the physiological pH of the gastrointestinal tract (1.2, 6.8 and 7.4) for 12 hours.
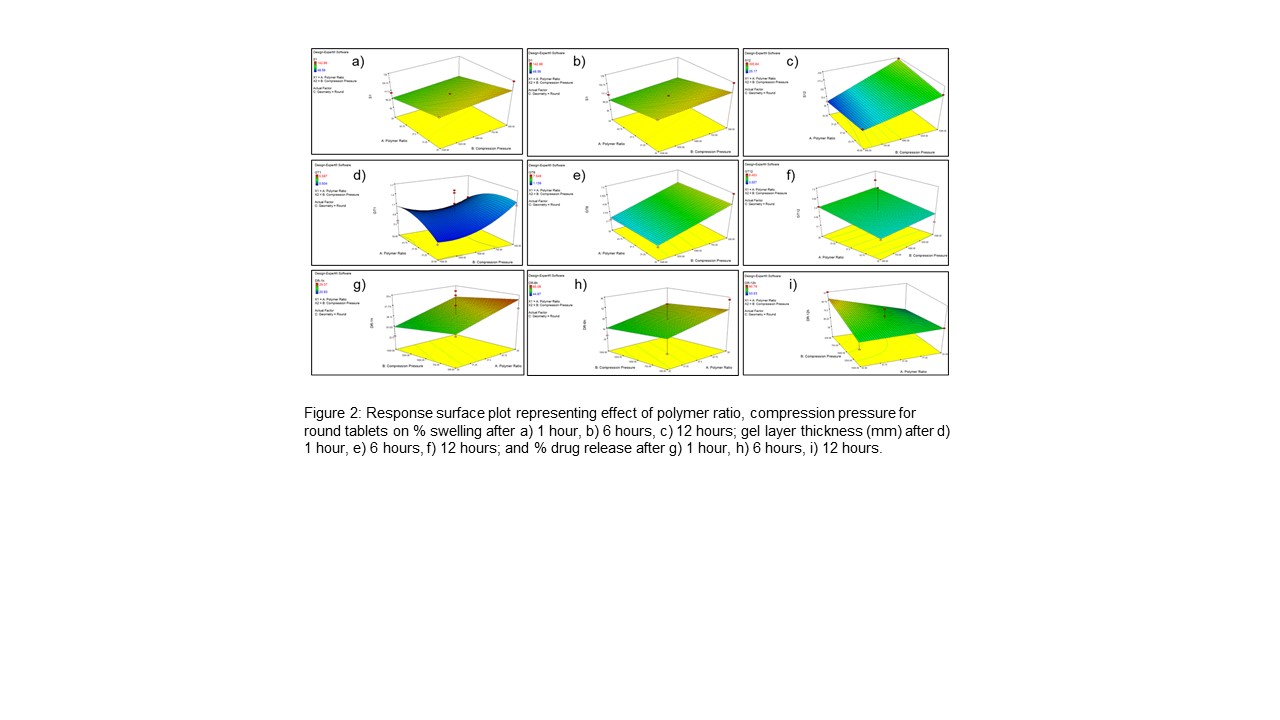
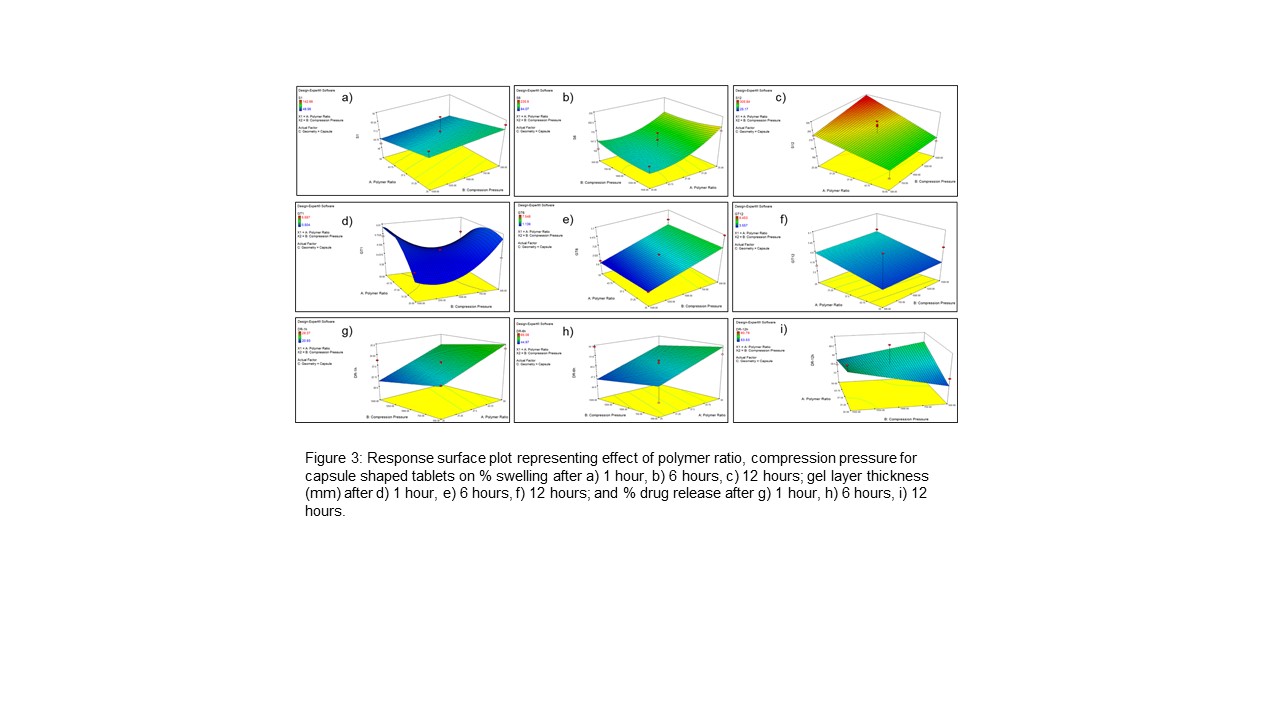
Results: The statistical relationship between independent and dependent variables was established using Design-Expert® statistical software. In this study, the % swelling of polymeric matrices was directly dependent on the compression pressure. With an increase in the compression pressure, the contact area between particles increased resulting in decreased porosity of matrix that obstructed solvent entry. With an increase in swelling, there was an increase in gel layer thickness around the matrix. Upon incorporation of Eudragit® RSPO with HPMC, there was an increase in the erosion rate of polymer matrix with an increase in the ratio of Eudragit® RSPO. This could be due to hydrogen bonding between the two polymers that altered the swelling and erosion of the polymer matrix. The effect of tablet geometry on swelling was due to the surface area of the matrix that encountered the dissolution medium. The capsule-shaped tablets had the smallest surface area and surface area/volume ratio followed by cylindrical and round tablets. Initially, regardless of tablet shape, the dissolution medium penetrates at the same rate. Polymer hydration leads to polymer relaxation and molecular rearrangement occurs and the formation of a gel layer takes place. For the first hour of dissolution, drug release was similar for all three shapes. The formation of the gel layer obstructed further infiltration of the dissolution medium to a deeper layer of tablet matrix and limited the subsequent release of the dissolved drug. Hence, the thickness of the gel layer was almost the same for different geometries, but the glassy core of tablets wasn’t identical. This glassy core needed to be hydrated for drug release and controlled the drug release. It was observed that after initial release, subsequent drug release was dependent on the surface area of the glassy core of the tablet that was influenced by tablet geometry. The round tablet shape with the largest surface area showed the highest drug release after 12 hours.
Conclusion: Experimental design was helpful in studying different factors affecting matrix hydration, erosion, gel layer formation, and ultimately drug release from hydrophilic polymer matrices. Major factors affecting drug release from hydrophilic matrix tablets are polymer type, process parameter, viz. compression pressure and tablet geometry. For a water-soluble drug, the release is dependent on the diffusion rate of the drug through the gel layer (diffusion layer) formed around matrix tablet because of water penetration. Drug release is also affected by tablet geometry which affects the surface area available for water penetration and drug release. Modulation of matrix shape alters the effective surface area and can be used as an approach to achieve the desired release rate.

Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(M1430-03-13) Investigation of Factors Influencing Drug Release from Controlled Release Hydrophilic Matrix Formulation using Central Composite Design of Experiments
Monday, October 17, 2022
2:30 PM – 3:30 PM ET
- SB
Shapali Bagde
St. John's University
Jamaica, New York, United States - SB
Shapali Bagde
St. John's University
Jamaica, New York, United States
Presenting Author(s)
Main Author(s)
Purpose: In hydrophilic polymeric matrix systems, drug is homogeneously dispersed in a rate-controlling polymer. There are several factors that affect drug release from these matrices, and knowledge about these factors and their influence is crucial for development of optimal formulation. When these matrices are placed in a dissolution medium, polymer hydrates and forms a gel diffusion layer which controls the release kinetics. Factors that influence formation and thickness of the diffusion layer determine rate of drug release. The type of polymer and its concentration determine the rate of water uptake, erosion of polymer, and thus formation of diffusional gel layer. The compression pressure is a critical process parameter affecting drug release. It governs number of pores matrix has for eluting fluid to penetrate and interact with polymer to form gel layer around matrix. Changing the geometry of the matrix tablets changes the surface area available for interaction with dissolution medium. In this study, an attempt was made to understand the factors, i.e. tablet geometry, the ratio of rate-controlling polymer in the matrix, and compression pressure, which influence the drug release from matrix system and optimize them using a central composite design.
Methods: In present study, a central composite design was used to study the effect of independent factors on dependent factors (responses). The experimental design consisted of three independent factors, viz. non-ionic:ionic polymer ratio (X1), compression pressure (X2), and tablet geometry (X3). These factors were studied at three levels, i.e. low, medium and high (-1, 0, +1). Tablet geometry was considered a categorical factor. Matrix tablets of cylindrical, round and capsule shapes were used in the study. The levels of independent factors for experimental designs were determined from initial screening experiments. Percent swelling, percent erosion, gel diffusion layer thickness, and drug release after 1, 6 and 12 hours were selected as dependent factors. Verapamil hydrochloride was selected as a model drug based on its high solubility in water. Tablets were prepared by direct compression of drug and polymer mixture in a 1:3 ratio. HPMC K4M and Eudragit® RSPO were selected as matrix-forming polymers. In order to record responses, dissolution, water uptake and erosion studies were conducted in dissolution media representing the physiological pH of the gastrointestinal tract (1.2, 6.8 and 7.4) for 12 hours.
Results: The statistical relationship between independent and dependent variables was established using Design-Expert® statistical software. In this study, the % swelling of polymeric matrices was directly dependent on the compression pressure. With an increase in the compression pressure, the contact area between particles increased resulting in decreased porosity of matrix that obstructed solvent entry. With an increase in swelling, there was an increase in gel layer thickness around the matrix. Upon incorporation of Eudragit® RSPO with HPMC, there was an increase in the erosion rate of polymer matrix with an increase in the ratio of Eudragit® RSPO. This could be due to hydrogen bonding between the two polymers that altered the swelling and erosion of the polymer matrix. The effect of tablet geometry on swelling was due to the surface area of the matrix that encountered the dissolution medium. The capsule-shaped tablets had the smallest surface area and surface area/volume ratio followed by cylindrical and round tablets. Initially, regardless of tablet shape, the dissolution medium penetrates at the same rate. Polymer hydration leads to polymer relaxation and molecular rearrangement occurs and the formation of a gel layer takes place. For the first hour of dissolution, drug release was similar for all three shapes. The formation of the gel layer obstructed further infiltration of the dissolution medium to a deeper layer of tablet matrix and limited the subsequent release of the dissolved drug. Hence, the thickness of the gel layer was almost the same for different geometries, but the glassy core of tablets wasn’t identical. This glassy core needed to be hydrated for drug release and controlled the drug release. It was observed that after initial release, subsequent drug release was dependent on the surface area of the glassy core of the tablet that was influenced by tablet geometry. The round tablet shape with the largest surface area showed the highest drug release after 12 hours.
Conclusion: Experimental design was helpful in studying different factors affecting matrix hydration, erosion, gel layer formation, and ultimately drug release from hydrophilic polymer matrices. Major factors affecting drug release from hydrophilic matrix tablets are polymer type, process parameter, viz. compression pressure and tablet geometry. For a water-soluble drug, the release is dependent on the diffusion rate of the drug through the gel layer (diffusion layer) formed around matrix tablet because of water penetration. Drug release is also affected by tablet geometry which affects the surface area available for water penetration and drug release. Modulation of matrix shape alters the effective surface area and can be used as an approach to achieve the desired release rate.



