Back
Purpose: Malignant melanoma is the deadliest form of skin cancer found to be most frequently associated with BRAF V600 mutations. Moreover, rapid resistance to BRAF inhibitors (BRAFi) like vemurafenib poses a major clinical problem and diverse pathways driving resistance converge to activate the transcription factor, c-Myc. This revelation enables the design of novel combination therapies that select against BRAFi resistance evolution in Myc-driven melanoma. Aurora kinase B and epigenetic reader BRD4 (bromo domain protein 4) are highly expressed in BRAFi resistant melanoma to subsequently increase the stability of oncogenic c-Myc for melanoma progression. Being promising therapeutic targets, we decided to combine a BRD4 targeted PROteolysis-TArgeting Chimera (ARV) with an Aurora kinase B inhibitor (AURKA) to drug the “undruggable” c-Myc in BRAFi-resistant melanoma. One step ahead, we developed ultra-deformable lipid vesicles called transfersomes to encapsulate ARV and AURKA (“Transfer-AA”) for their enhanced skin penetration and transdermal delivery for melanoma therapy (Fig. 1a). Our research objectives were; (a) to identify the synergism between ARV and AURKA in 2D and 3D BRAFi-resistant melanoma models, and (b) to develop and characterize ARV and AURKA encapsulated transfersomes (“Transfer-AA”).
Methods: Cytotoxic activity of ARV and AURKA was analyzed in parent (SK-MEL-28 and A375) and vemurafenib-resistant (SV, AV and RPMI-7951) melanoma cell lines using MTT assay. ARV-AURKA combination assay was carried out at different molar ratios to evaluate the synergistic interaction between both the drugs in vemurafenib-resistant melanoma cell lines and the combination index values were calculated using Chou–Talalay method. In vitro migration and clonogenic assays were performed for ARV, AURKA and their combination in resistant melanoma cells. Annexin V assay was used to determine the apoptotic effect of ARV and AURKA in resistant melanoma cell lines which was further analyzed using flow cytometry. To better mimic the in vivo tumor microenvironment, we evaluated the growth, apoptosis, and invasion of 3D multicellular AV spheroids following ARV and AURKA treatment. Transfer-AA were developed and optimized by thin film hydration method using DOPC, cholesterol and DSPE-PEG lipids (15:2.5:1 weight ratio) with incorporation of Tween-80 as an edge activator at 10 %w/w and 20 %w/w of total lipids to form ultra-deformable lipid vesicles (Fig. 3a). ARV (1 mg/mL) and AURKA (3 mg/mL) were encapsulated in Transfer-AA followed by its physicochemical characterization in terms of particle size, polydispersity index, zeta potential, drugs entrapment efficiency, and deformability index.
Results: ARV and AURKA revealed strong synergism at molar ratio of 1:5 in vemurafenib-resistant melanoma cell lines with the lowest combination index value of 0.46 ± 0.07 in AV cells (Fig. 1b-c). Combination of ARV and AURKA also substantially inhibited the in vitro migration and clonogenic potential of resistant melanoma cell lines (Fig. 1d-e). Annexin V assay results demonstrated significant apoptotic effect mediated by this synergistic combination of ARV and AURKA suggesting its strong antiproliferative activity in resistant melanoma cells (Fig. 1f). Similar combination of ARV and AURKA led to significant reduction in the area and invasiveness of 3D AV spheroids with large apoptotic population as depicted by their bright red fluorescence (Fig. 2a-c, e). Interestingly, the length of invadopodia formed and percent invasion in 3D AV spheroids was substantially lower for the combination treatment as compared to the individual drugs or control group (Fig. 2d). Formulated conventional liposomes and transfersomes yielded size of < 100 nm, unimodal size distribution and neutral surface charge. Further, incorporation of both the drugs in Transfer-AA with 20% w/w Tween-80 resulted in 73.2% and 84.1% entrapment efficiency of ARV and AURKA, respectively and a deformability index of 1.04 indicating the formation of ultra-deformable lipid vesicles (Fig. 3b-c). Ex vivo skin permeation and drug retention of Transfer-AA will be studied using Franz diffusion cells and Strat-M® membrane to identify permeation and skin retention of ARV and AURKA via Transfer-AA for their transdermal delivery. Drugs permeated and retained in skin will then be quantified using developed and validated HPLC method. In vitro cytotoxicity evaluation and western blot analysis for expression of BRD4, c-Myc, cleaved caspase-3 and Arora kinase B after treatment with Transfer-AA in AV cells is currently under investigation.
Conclusion: Our results demonstrate that combination of BRD4 PROTAC and Aurora Kinase B inhibitor leads to synergistic antitumor effects against BRAF inhibitor-resistant melanoma. Development of ultra-deformable “Transfer-AA” further augments the efficacy of this treatment cocktail for its successful transdermal delivery in BRAF inhibitor-resistant melanoma therapy.
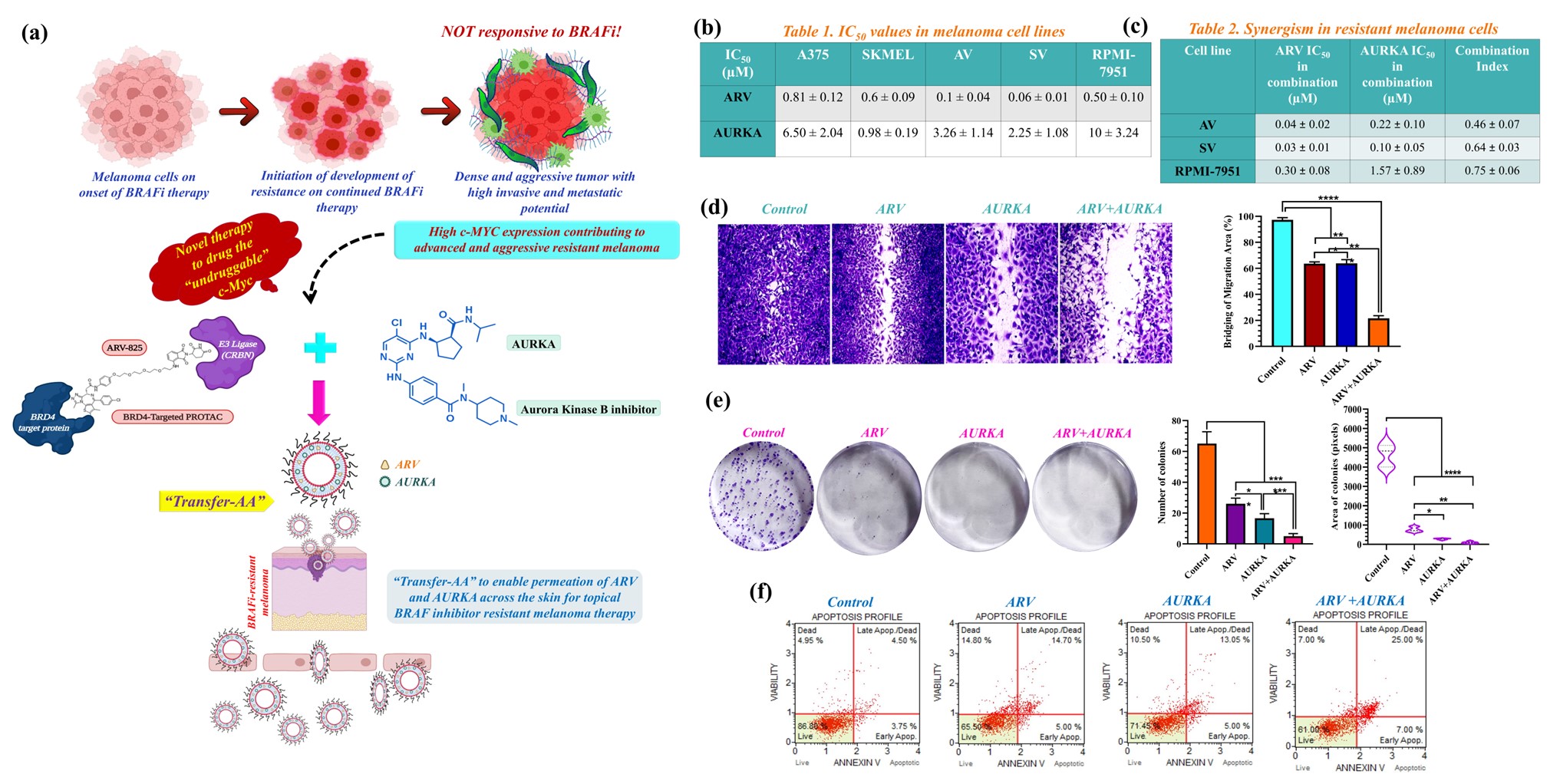
Fig. 1. (a) A novel synergistic combination of ARV and AURKA encapsulated in “Transfer-AA” for its transdermal delivery in BRAFi resistant malignant melanoma therapy. (b-c) Cytotoxic interaction of ARV and AURKA alone and in combination (1:5 molar ratio) in parent and BRAFi-resistant melanoma cells. (d) In vitro migration and (e) Clonogenic assay results in AV cells. (f) Apoptosis assay results of ARV and AURKA treated AV cells. Data are expressed as mean ± S.D. *, p < 0.05, **, p < 0.01, ***, p < 0.001, ****, p < 0.0001.
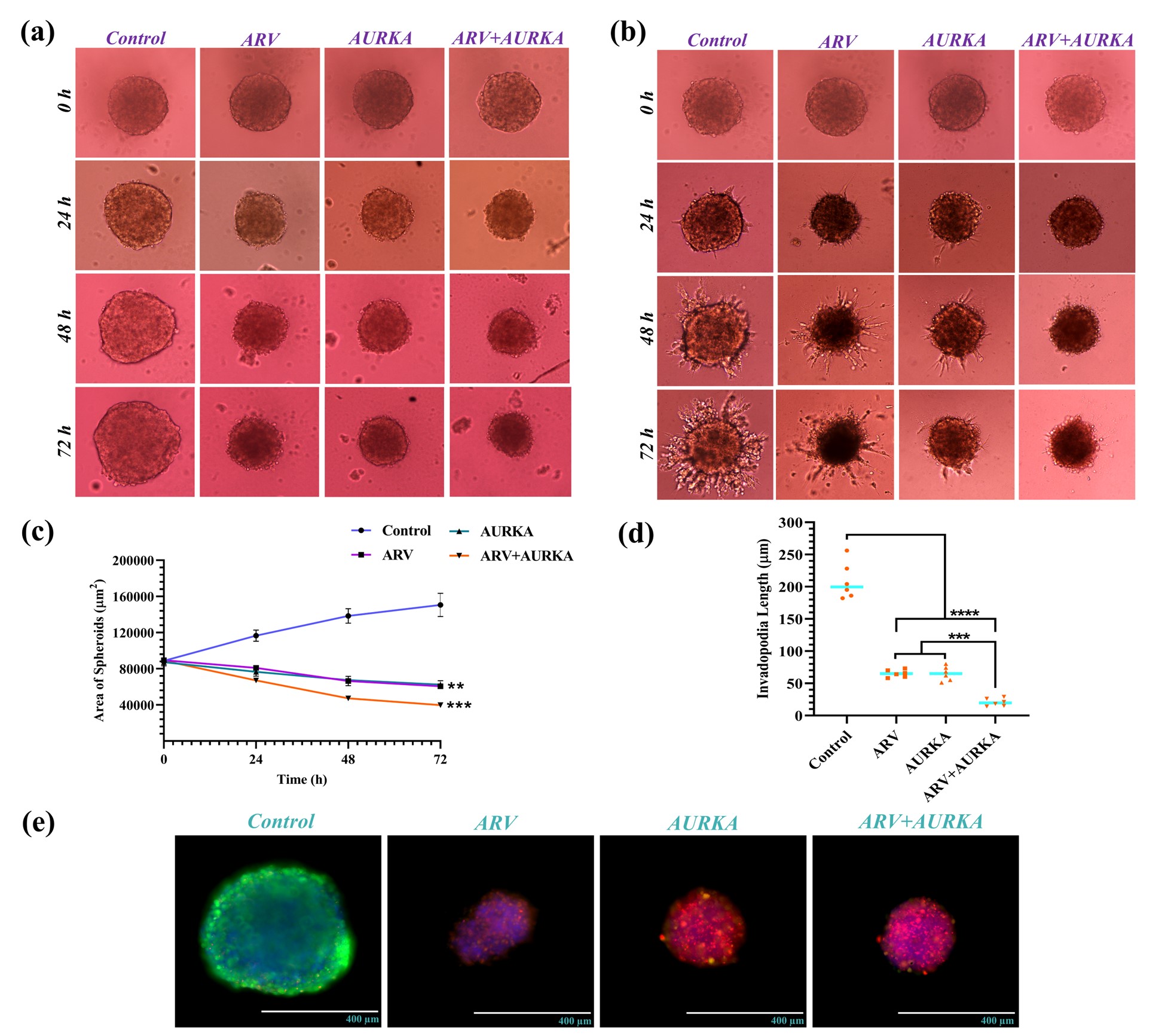
Fig. 2. (a-b) Spheroid growth and invasion in ARV and AURKA-treated 3D multicellular AV spheroids. (c) Growth curve for 3D AV spheroids following ARV and AURKA treatment alone and in combination. (d) Combination of ARV and AURKA depicted substantial inhibition in invadopodia length of 3D multicellular AV spheroids. (e) Results of cell viability within ARV and AURKA-treated 3D multicellular AV spheroids. Data are expressed as mean ± S.D. *, p < 0.05, **, p < 0.01, ***, p < 0.001, ****, p < 0.0001.
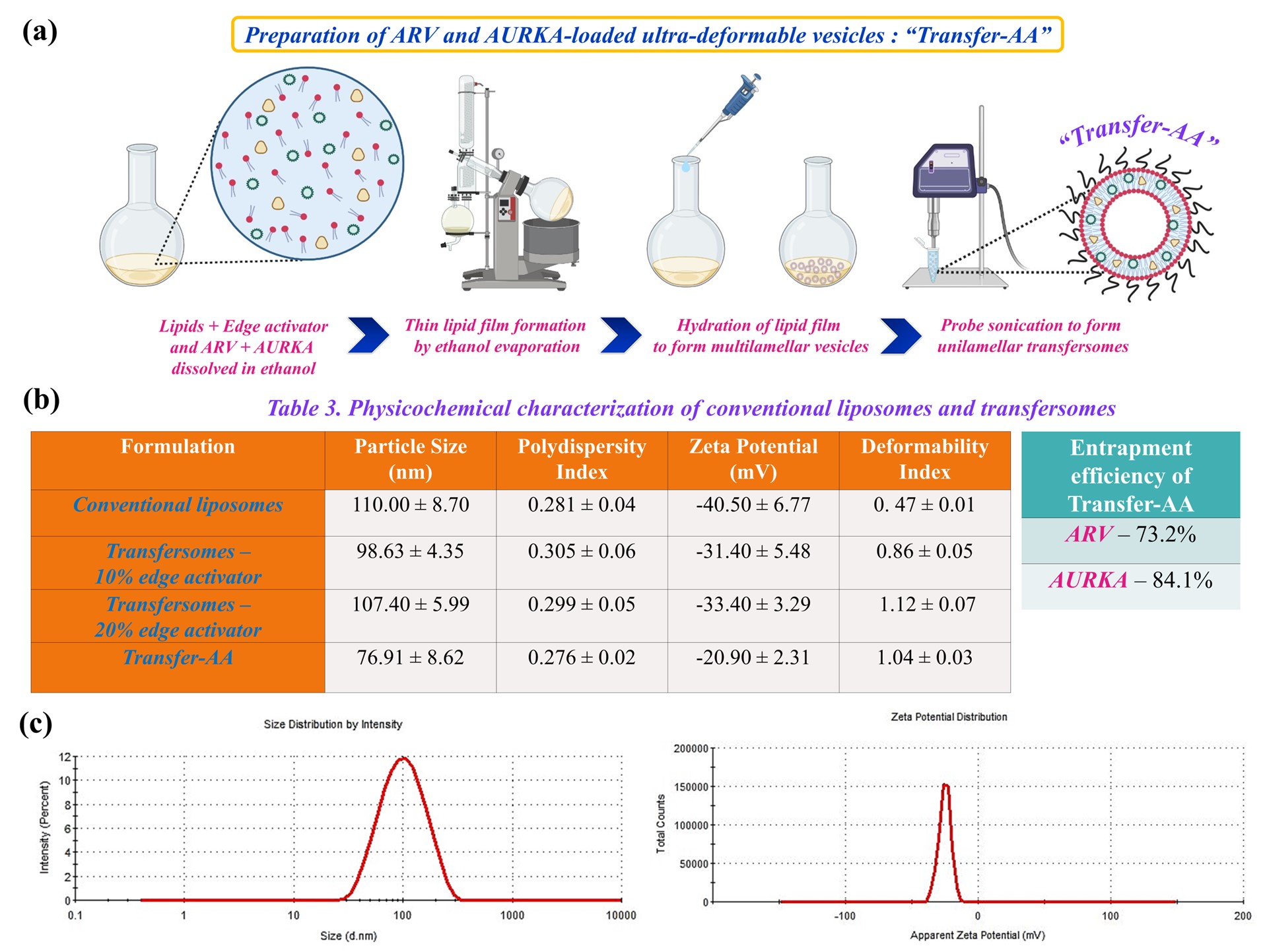
Fig. 3. (a) Schematic representation demonstrating preparation of Transfer-AA by thin film hydration method. (b) Physicochemical characterization of different formulation batches and optimized Transfer-AA. (c) Dynamic light scattering graphs depicting uniform particle size and surface charge of Transfer-AA.
Formulation and Delivery - Biomolecular - Drug Delivery
Category: Poster Abstract
(M1230-07-40) Ultra-deformable Nanocarrier of BRD4 PROTAC and Aurora Kinase B Inhibitor to Target “Undruggable” c-Myc in BRAF Inhibitor-Resistant Melanoma
Monday, October 17, 2022
12:30 PM – 1:30 PM ET
- AS
Aishwarya Saraswat, MS
St. John's University
Jamaica, New York, United States - AS
Aishwarya Saraswat, MS
St. John's University
Jamaica, New York, United States
Presenting Author(s)
Main Author(s)
Purpose: Malignant melanoma is the deadliest form of skin cancer found to be most frequently associated with BRAF V600 mutations. Moreover, rapid resistance to BRAF inhibitors (BRAFi) like vemurafenib poses a major clinical problem and diverse pathways driving resistance converge to activate the transcription factor, c-Myc. This revelation enables the design of novel combination therapies that select against BRAFi resistance evolution in Myc-driven melanoma. Aurora kinase B and epigenetic reader BRD4 (bromo domain protein 4) are highly expressed in BRAFi resistant melanoma to subsequently increase the stability of oncogenic c-Myc for melanoma progression. Being promising therapeutic targets, we decided to combine a BRD4 targeted PROteolysis-TArgeting Chimera (ARV) with an Aurora kinase B inhibitor (AURKA) to drug the “undruggable” c-Myc in BRAFi-resistant melanoma. One step ahead, we developed ultra-deformable lipid vesicles called transfersomes to encapsulate ARV and AURKA (“Transfer-AA”) for their enhanced skin penetration and transdermal delivery for melanoma therapy (Fig. 1a). Our research objectives were; (a) to identify the synergism between ARV and AURKA in 2D and 3D BRAFi-resistant melanoma models, and (b) to develop and characterize ARV and AURKA encapsulated transfersomes (“Transfer-AA”).
Methods: Cytotoxic activity of ARV and AURKA was analyzed in parent (SK-MEL-28 and A375) and vemurafenib-resistant (SV, AV and RPMI-7951) melanoma cell lines using MTT assay. ARV-AURKA combination assay was carried out at different molar ratios to evaluate the synergistic interaction between both the drugs in vemurafenib-resistant melanoma cell lines and the combination index values were calculated using Chou–Talalay method. In vitro migration and clonogenic assays were performed for ARV, AURKA and their combination in resistant melanoma cells. Annexin V assay was used to determine the apoptotic effect of ARV and AURKA in resistant melanoma cell lines which was further analyzed using flow cytometry. To better mimic the in vivo tumor microenvironment, we evaluated the growth, apoptosis, and invasion of 3D multicellular AV spheroids following ARV and AURKA treatment. Transfer-AA were developed and optimized by thin film hydration method using DOPC, cholesterol and DSPE-PEG lipids (15:2.5:1 weight ratio) with incorporation of Tween-80 as an edge activator at 10 %w/w and 20 %w/w of total lipids to form ultra-deformable lipid vesicles (Fig. 3a). ARV (1 mg/mL) and AURKA (3 mg/mL) were encapsulated in Transfer-AA followed by its physicochemical characterization in terms of particle size, polydispersity index, zeta potential, drugs entrapment efficiency, and deformability index.
Results: ARV and AURKA revealed strong synergism at molar ratio of 1:5 in vemurafenib-resistant melanoma cell lines with the lowest combination index value of 0.46 ± 0.07 in AV cells (Fig. 1b-c). Combination of ARV and AURKA also substantially inhibited the in vitro migration and clonogenic potential of resistant melanoma cell lines (Fig. 1d-e). Annexin V assay results demonstrated significant apoptotic effect mediated by this synergistic combination of ARV and AURKA suggesting its strong antiproliferative activity in resistant melanoma cells (Fig. 1f). Similar combination of ARV and AURKA led to significant reduction in the area and invasiveness of 3D AV spheroids with large apoptotic population as depicted by their bright red fluorescence (Fig. 2a-c, e). Interestingly, the length of invadopodia formed and percent invasion in 3D AV spheroids was substantially lower for the combination treatment as compared to the individual drugs or control group (Fig. 2d). Formulated conventional liposomes and transfersomes yielded size of < 100 nm, unimodal size distribution and neutral surface charge. Further, incorporation of both the drugs in Transfer-AA with 20% w/w Tween-80 resulted in 73.2% and 84.1% entrapment efficiency of ARV and AURKA, respectively and a deformability index of 1.04 indicating the formation of ultra-deformable lipid vesicles (Fig. 3b-c). Ex vivo skin permeation and drug retention of Transfer-AA will be studied using Franz diffusion cells and Strat-M® membrane to identify permeation and skin retention of ARV and AURKA via Transfer-AA for their transdermal delivery. Drugs permeated and retained in skin will then be quantified using developed and validated HPLC method. In vitro cytotoxicity evaluation and western blot analysis for expression of BRD4, c-Myc, cleaved caspase-3 and Arora kinase B after treatment with Transfer-AA in AV cells is currently under investigation.
Conclusion: Our results demonstrate that combination of BRD4 PROTAC and Aurora Kinase B inhibitor leads to synergistic antitumor effects against BRAF inhibitor-resistant melanoma. Development of ultra-deformable “Transfer-AA” further augments the efficacy of this treatment cocktail for its successful transdermal delivery in BRAF inhibitor-resistant melanoma therapy.

Fig. 1. (a) A novel synergistic combination of ARV and AURKA encapsulated in “Transfer-AA” for its transdermal delivery in BRAFi resistant malignant melanoma therapy. (b-c) Cytotoxic interaction of ARV and AURKA alone and in combination (1:5 molar ratio) in parent and BRAFi-resistant melanoma cells. (d) In vitro migration and (e) Clonogenic assay results in AV cells. (f) Apoptosis assay results of ARV and AURKA treated AV cells. Data are expressed as mean ± S.D. *, p < 0.05, **, p < 0.01, ***, p < 0.001, ****, p < 0.0001.

Fig. 2. (a-b) Spheroid growth and invasion in ARV and AURKA-treated 3D multicellular AV spheroids. (c) Growth curve for 3D AV spheroids following ARV and AURKA treatment alone and in combination. (d) Combination of ARV and AURKA depicted substantial inhibition in invadopodia length of 3D multicellular AV spheroids. (e) Results of cell viability within ARV and AURKA-treated 3D multicellular AV spheroids. Data are expressed as mean ± S.D. *, p < 0.05, **, p < 0.01, ***, p < 0.001, ****, p < 0.0001.

Fig. 3. (a) Schematic representation demonstrating preparation of Transfer-AA by thin film hydration method. (b) Physicochemical characterization of different formulation batches and optimized Transfer-AA. (c) Dynamic light scattering graphs depicting uniform particle size and surface charge of Transfer-AA.
