Back
Purpose: Malignant pleural mesothelioma is lethal neoplasm arising from mesothelial cells lining the pleura, peritoneum, tunica vaginalis testis and pericardium. Currently, there is no cure, and patients relapse after surgery. In this context, we proposed Hyalcis, a cisplatin/hyaluronan film, as a novel dosage form for loco-regional drug delivery to be used in mesothelioma and in similar tumors developing in a body cavity. Long term physicochemical stability is very imperative for pharmaceutical dosage form. We observed anomaly in vitro release behavior with the time. In particular, the amount of NaHA dissolved from film over time significantly decreased with film age, while CisPt release remained unchanged. Hence, the aim of the present study was to systematically investigate stability/incompatibility issue and understand any underling mechanism causing reduced solubility of hyaluronan.
Methods: Batches with different compositions of cisplatin/hyaluronan films (Table I) were prepared by layering and drying the corresponding aqueous film-forming mixtures. Then, dry films were stored at 40±2 °C and 75±5% RH for 6 months. Films were regularly characterized by solid state using scanning calorimetry (DSC), powder X-ray diffractometry, and polarized light microscopy (PLM). Film tensile strength was measured using a texture analyzer (TA.XTPlus). Moisture sorption on polymer raw material and films were carried out using VTI SA dynamic vapor sorption analyzer. CisPt release from film was determined in 0.9% NaCl solution (pH 7.0±0.1) at 37 °C under magnetic stirring for up to 96h. Simultaneous quantification of NaHA and CisPt in release samples was carried out by reverse phase HPLC (RP-HPLC). The release samples were also analyzed by Dynamic Light Diffraction (DLS) for colloidal property.
Results: Films of uniform thickness were prepared, and their appearance was transparent. Clarity and no white opaqueness confirmed that no phase separation occurred during manufacturing. DSC, XRD and PLM analyses allowed to determine the drug–excipients miscibility and system stability, which are critical for successful development of the final product. DSC scans of neat excipients (not shown), drug and films are shown in Figure 1 (A). Storage conditions do not affect the DSC profile of films but there is a difference noticed on day 1 compared to day 30. This would be further investigated. The powder XRD patterns show that all films are semi-crystalline on day 1 and after 1 month, indicating no change in crystallinity. Polarized microscopy images of the films formed from different drug–polymer mixtures, recorded within 24 h of film layering and after exposure at 40°C/75% RH for 15 (images not shown) and 30 days, are shown in Figure 1 (B). They confirm no drug crystallization, however the film morphology changed substantially. Tensile strength, measured at day 1, of placebo film (2.29±0.40 kg/mm2) was greater than the other compositions, except composition D (Figure 2). Moisture sorption analysis showed that CisPt-loaded film (composition A) can absorb twice as much moisture as compared to drug-free film (35% vs. 17%).
Cisplatin release from fresh film was the same for all compositions, with about 85% of CisPt released in 48h. During the release from films of composition C, an interaction of the vial with light gave a blue reflection, suggesting the formation of colloidal structures. DLS analysis of these samples evidenced the presence of nanoparticles with a mean diameter of 102.6 ± 20.85 nm and PDI of 0.338 ± 0.12. Zeta potential was negative, as expected due to the anionic polymer.
Conclusion: Overall, the study is still ongoing with the aim to verify the film stability of film under accelerated stress conditions. Cisplatin release from fresh film was not affected by the change in the composition. CisPt release and NaHA dissolution from aged film in accelerated stability conditions would be further investigated.
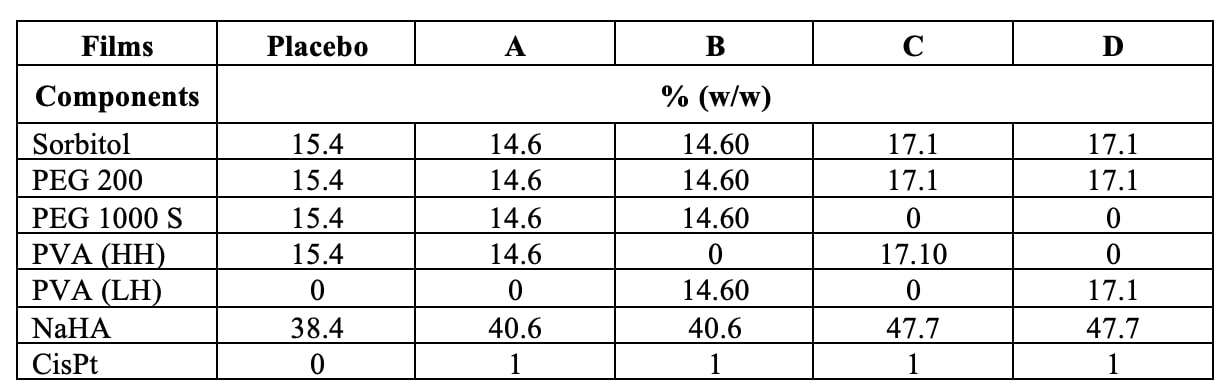
Table I. Hyaluronate films composition by dry weight (% w/w).
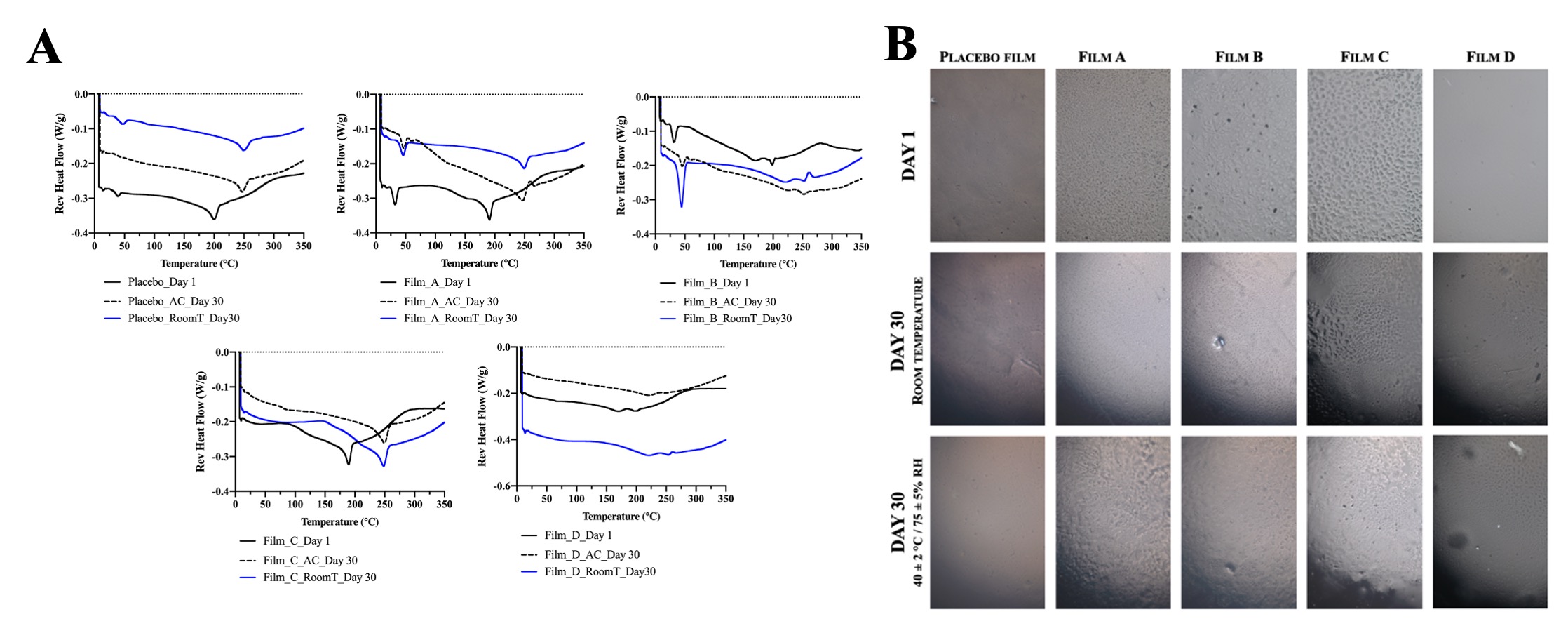
Figure 1. (A) Differential scanning calorimetry (DSC) scans of the films freshly prepared and after exposure to 40 °C/75% RH (accelerated stability, AC) and to room temperature (RoomT) for 1 month. (B) Polarized light microscopic images (10x) of the films at day 1 and after 1 month at room temperature and under accelerated stress conditions (402 °C / 755% RH).
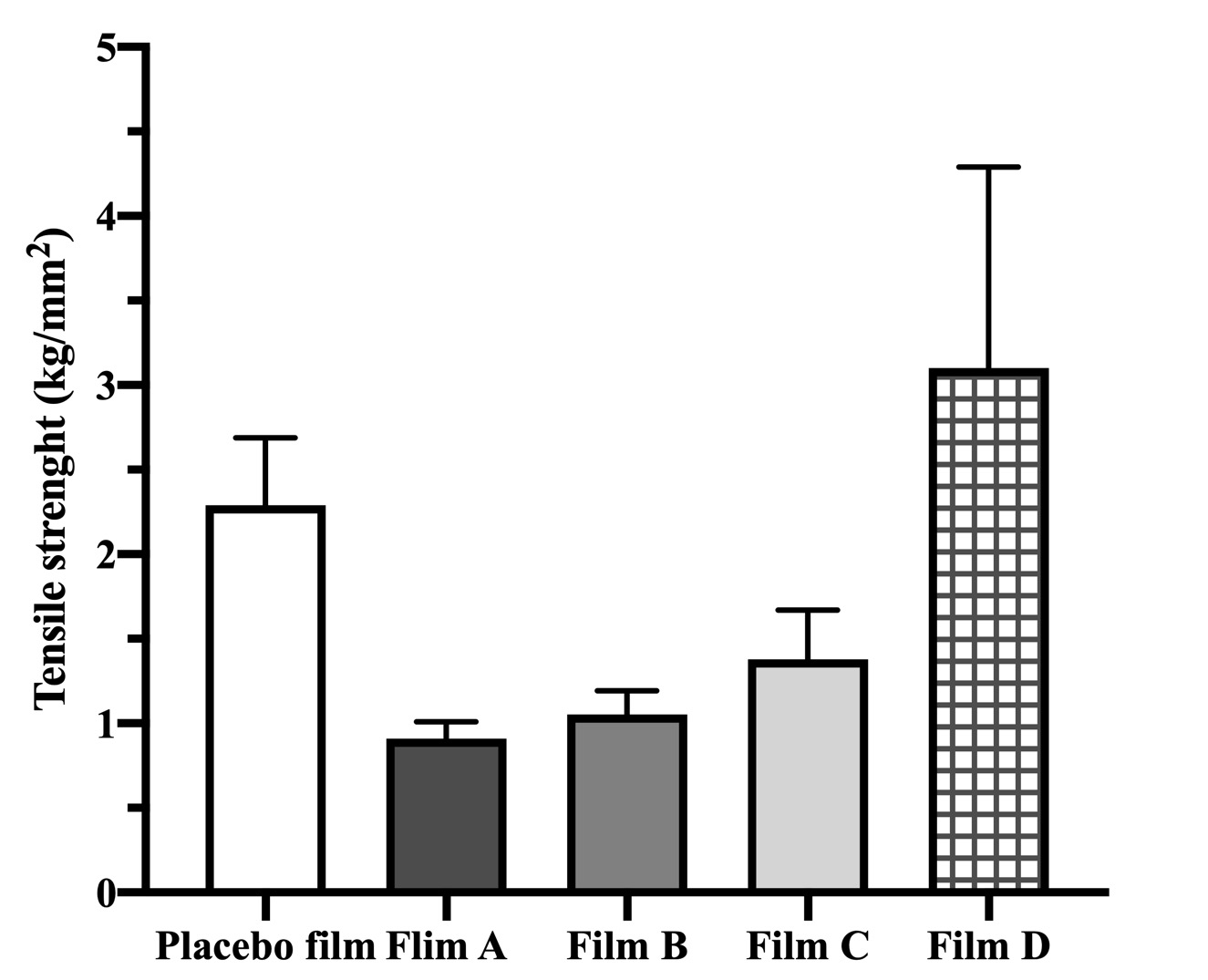
Figure 2. Tensile strength of the films freshly prepared (Day 1).
Formulation and Delivery - Chemical - Drug Delivery
Category: Poster Abstract
(M1130-04-19) Stability and Optimization Study of Cisplatin/Hyaluronan Film for Loco-Regional Drug Delivery in Cancer
Monday, October 17, 2022
11:30 AM – 12:30 PM ET
- SB
Sabrina Banella
University of Ferrara
Ferrara, Emilia-Romagna, Italy - SB
Sabrina Banella
University of Ferrara
Ferrara, Emilia-Romagna, Italy
Presenting Author(s)
Main Author(s)
Purpose: Malignant pleural mesothelioma is lethal neoplasm arising from mesothelial cells lining the pleura, peritoneum, tunica vaginalis testis and pericardium. Currently, there is no cure, and patients relapse after surgery. In this context, we proposed Hyalcis, a cisplatin/hyaluronan film, as a novel dosage form for loco-regional drug delivery to be used in mesothelioma and in similar tumors developing in a body cavity. Long term physicochemical stability is very imperative for pharmaceutical dosage form. We observed anomaly in vitro release behavior with the time. In particular, the amount of NaHA dissolved from film over time significantly decreased with film age, while CisPt release remained unchanged. Hence, the aim of the present study was to systematically investigate stability/incompatibility issue and understand any underling mechanism causing reduced solubility of hyaluronan.
Methods: Batches with different compositions of cisplatin/hyaluronan films (Table I) were prepared by layering and drying the corresponding aqueous film-forming mixtures. Then, dry films were stored at 40±2 °C and 75±5% RH for 6 months. Films were regularly characterized by solid state using scanning calorimetry (DSC), powder X-ray diffractometry, and polarized light microscopy (PLM). Film tensile strength was measured using a texture analyzer (TA.XTPlus). Moisture sorption on polymer raw material and films were carried out using VTI SA dynamic vapor sorption analyzer. CisPt release from film was determined in 0.9% NaCl solution (pH 7.0±0.1) at 37 °C under magnetic stirring for up to 96h. Simultaneous quantification of NaHA and CisPt in release samples was carried out by reverse phase HPLC (RP-HPLC). The release samples were also analyzed by Dynamic Light Diffraction (DLS) for colloidal property.
Results: Films of uniform thickness were prepared, and their appearance was transparent. Clarity and no white opaqueness confirmed that no phase separation occurred during manufacturing. DSC, XRD and PLM analyses allowed to determine the drug–excipients miscibility and system stability, which are critical for successful development of the final product. DSC scans of neat excipients (not shown), drug and films are shown in Figure 1 (A). Storage conditions do not affect the DSC profile of films but there is a difference noticed on day 1 compared to day 30. This would be further investigated. The powder XRD patterns show that all films are semi-crystalline on day 1 and after 1 month, indicating no change in crystallinity. Polarized microscopy images of the films formed from different drug–polymer mixtures, recorded within 24 h of film layering and after exposure at 40°C/75% RH for 15 (images not shown) and 30 days, are shown in Figure 1 (B). They confirm no drug crystallization, however the film morphology changed substantially. Tensile strength, measured at day 1, of placebo film (2.29±0.40 kg/mm2) was greater than the other compositions, except composition D (Figure 2). Moisture sorption analysis showed that CisPt-loaded film (composition A) can absorb twice as much moisture as compared to drug-free film (35% vs. 17%).
Cisplatin release from fresh film was the same for all compositions, with about 85% of CisPt released in 48h. During the release from films of composition C, an interaction of the vial with light gave a blue reflection, suggesting the formation of colloidal structures. DLS analysis of these samples evidenced the presence of nanoparticles with a mean diameter of 102.6 ± 20.85 nm and PDI of 0.338 ± 0.12. Zeta potential was negative, as expected due to the anionic polymer.
Conclusion: Overall, the study is still ongoing with the aim to verify the film stability of film under accelerated stress conditions. Cisplatin release from fresh film was not affected by the change in the composition. CisPt release and NaHA dissolution from aged film in accelerated stability conditions would be further investigated.

Table I. Hyaluronate films composition by dry weight (% w/w).

Figure 1. (A) Differential scanning calorimetry (DSC) scans of the films freshly prepared and after exposure to 40 °C/75% RH (accelerated stability, AC) and to room temperature (RoomT) for 1 month. (B) Polarized light microscopic images (10x) of the films at day 1 and after 1 month at room temperature and under accelerated stress conditions (402 °C / 755% RH).

Figure 2. Tensile strength of the films freshly prepared (Day 1).
