Back
Purpose: Lung cancer is the leading cause of cancer deaths globally, with most of the reported cases ( >85%) associated with non-small cell lung cancer (NSCLC). The available chemotherapy and immunotherapy options often lack tumor-targeting leading to systemic toxicity and eventual development of drug resistance, limiting the choice of cancer therapies. Antimicrobial peptides (AMPs) have gained interest as anticancer agents as they selectively target cancer cells and decrease the possibility of resistance. Nisin ZP, an antimicrobial peptide (AMP) produced by the bacterium Lactococcus lactis, has demonstrated anticancer activity in preclinical reports. In this study, we evaluated the anticancer potential and underlying mechanisms of nisin ZP in NSCLC cells. Also, the delivery of peptides by conventional route (oral and parenteral) results in low drug concentration in the lungs where tumor burden is high. Hence, we formulated a nisin ZP dry powder using spray-dryer (NZSD) to facilitate inhaled delivery and eventual high drug concentration at the target site.
Methods: The in vitro cytotoxicity was evaluated using MTT assay in NSCLC (A549) and healthy (HEK293) cells. In addition, kit-based apoptosis, cell cycle, mitochondrial membrane potential, and reactive oxygen species (ROS) assays were performed to determine the mechanism of action. The wound healing, clonogenic, and spheroid assays (volume analysis and spheroid viability) were conducted to support the application of nisin ZP in NSCLC. Nisin ZP (30% w/w) was spray-dried with mannitol, L-leucine, and trehalose in an optimized ratio (75:15:10) using Büchi mini spray-dryer B-290. The spray-dried powder was characterized for yield, peptide content, residual moisture content, solid-state characteristics (Differential Scanning Calorimetry; DSC and Powder X-Ray Diffraction; PXRD) and morphology (Scanning Electron Microscopy; SEM). Aerosolization performance of dry powders was evaluated using Plastiape RS01 dry powder inhaler device in Next Generation Impactor (NGI™️). The stability studies of the dry powder were performed after storage at refrigerated (4 ºC) and room temperature (25 ºC).
Results: Nisin ZP induced selective toxicity in cancer (A549; IC50 132.4 ± 6.4 µM) cells compared to healthy (HEK293; IC50 >300 µM) cells after 48 h of treatment (fig. 1a). Nisin ZP exposure to A549 cells activated the apoptotic pathway and arrested cell cycle progression in the initial G0/G1 growth phase (fig. 1b & c). The cancer cell proliferation was inhibited via non-membranolytic pathways by mitochondrial membrane depolarization (reduction in potential; fig. 1d) and elevation in intracellular ROS levels in a dose-dependent manner (fig. 1e). The clonal expansion (reduction in colony formation; fig. 2a) and wound healing (restricted wound closure; fig 2b) of cancer cells were inhibited by nisin ZP treatment suggesting potential application against highly metastatic NSCLC. In 3D spheroid assay, the multi-dose regimen of nisin ZP at 250 µM (0.071 ± 0.005 mm3) limited the tumor growth compared to control (0.116 ± 0.009 mm3) and 125 µM (0.113 ± 0.007 mm3) treatment group after 12 days (fig. 2d & e), suggesting excellent antitumor potential at twice inhibitory concentration compared to cell monolayer-based assays. This result was further supported by spheroid cell viability, demonstrating significant inhibition in viability for nisin ZP (250 µM) treatment group (fig. 2c).
NZSD formulation revealed a good powder yield of 62.0 ± 4.2% w/w, high peptide content of 326.6 ± 16.3 µg/mg, and low residual moisture content ( < 3.0% w/w; fig. 3a). The presence of an endothermic peak in DSC thermograms (fig. 3b) and attenuated crystalline peaks in PXRD diffractograms (fig. 3c) confirmed the semi-crystalline powder nature with smooth spherical morphology. Aerosolization studies performed using NGI indicated an aerodynamic diameter of 1.12 ± 0.11 μm within the desired range (1-5 µm) and a high fine particle fraction of 92.19 ± 0.71% (fig. 3d), suggesting deposition of powder in respiratory airways of the lung. The anticancer activity of nisin ZP was maintained after fabricating it into NZSD powder and showed a similar inhibitory concentration (fig. 3e) to nisin ZP. Dry powder was stable at both the storage conditions (refrigerated and room temperature) with 95% drug content and semi-crystalline nature, as confirmed by DSC and PXRD studies (data not shown) for three months.
Conclusion: Nisin ZP inhibited lung cancer cell proliferation via the non-membranolytic pathway and exhibited excellent anticancer efficacy against advanced NSCLC in vitro. Further, spray-dried nisin ZP formulation resulted in free-flowing dry powder, enhanced physical stability, and enabled inhaled delivery.
Acknowledgements:This work was funded by the American Association of Colleges of Pharmacy (AACP) via New Investigator Award to Nitesh K. Kunda.
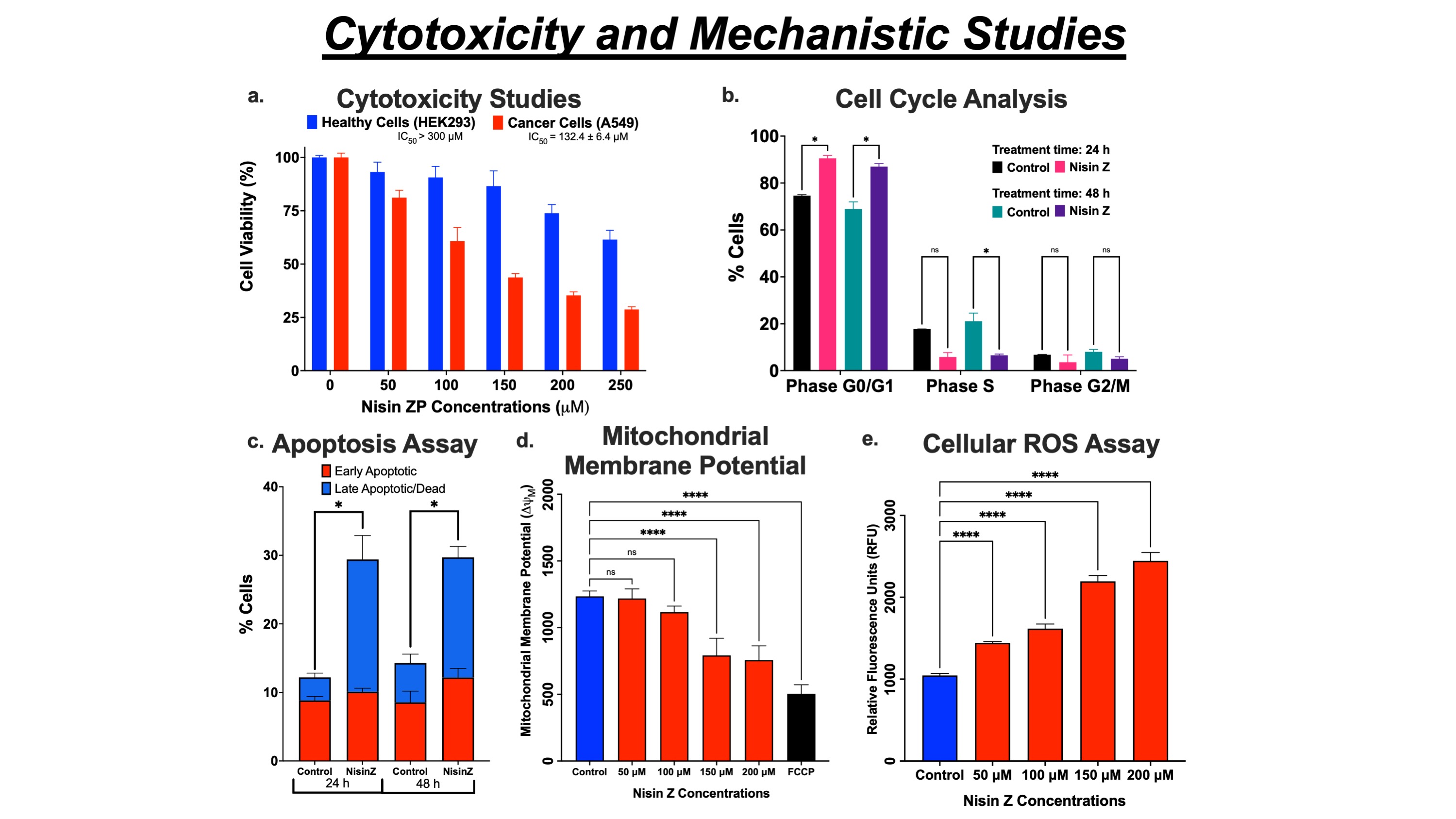
Figure 1. Cytotoxicity and Mechanistic Studies. a. Cytotoxicity (MTT assay) results following Nisin ZP treatment for 48 h in healthy (HEK293) and cancer (A549) Cells. b. Cell cycle arrest of NSCLC cells in G0/G1 phase after nisin ZP treatment in A549 cells. c. Nisin ZP induces apoptosis in NSCLC cells after 24 and 48 h treatment. d. The mitochondrial membrane's polarization is indicated by a decrease in ΔΨM of NSCLC (A549) cells. e. Elevation in intracellular ROS levels following nisin ZP treatment.
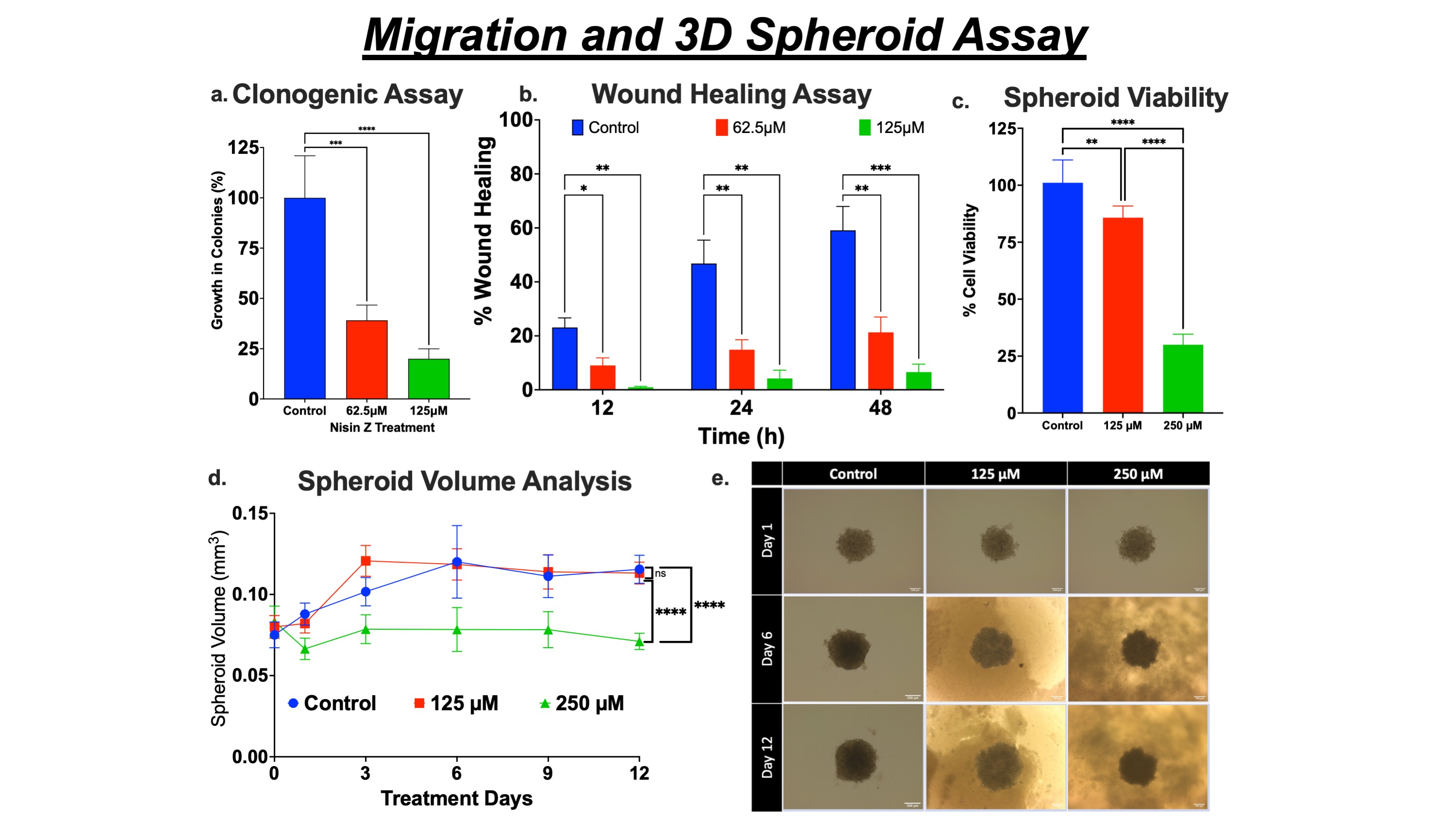
Figure 2. Migration and 3D Spheroid Assay. a. Nisin ZP inhibits the colony formation in NSCLC cells, reported as percent growth in colonies. b. Nisin ZP inhibits the metastatic activity of NSCLC cells, shown as % wound healing over time. c. Cell viability assay of 3D spheroids suggesting significant inhibition. d. Spheroid volume growth curve of A549 cells after multiple doses of nisin ZP. e. Representative microscopic images of spheroids.
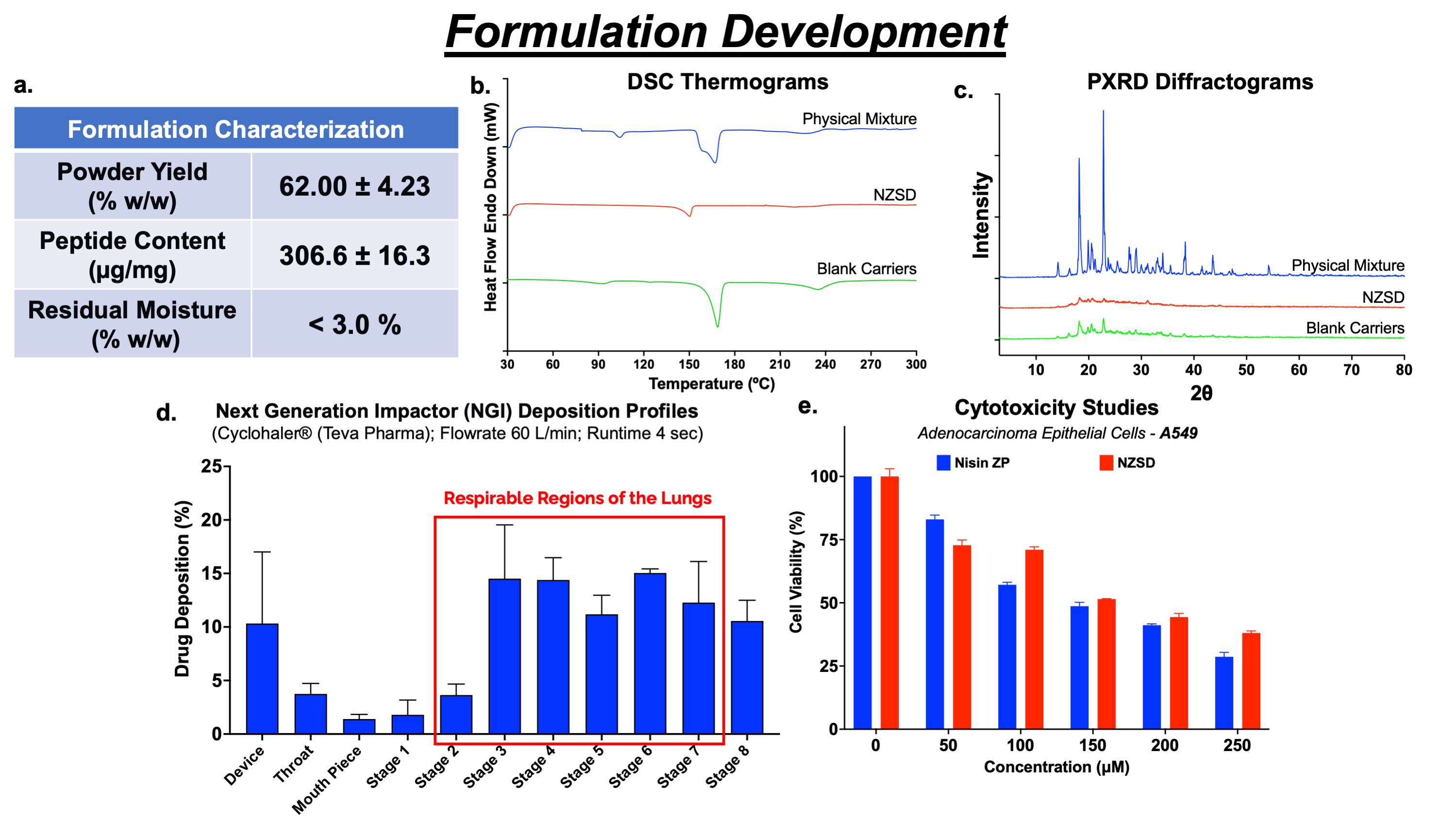
Figures 3. Spray-dried Nisin ZP formulation development. a. Formulation characteristics of NZSD dry powder. Solid-state characterization of powders - b. Differential Scanning Calorimetry (DSC) Thermograms c. Powder X-Ray Diffraction Studies suggesting semi-crystalline nature. d. Aerosolization performance of NZSD analyzed by drug deposition on various stages of Next Generation Impactor (NGI). e. Cytotoxicity studies (MTT Assay) of nisin ZP and NZSD demonstrated similar anticancer activity.
Formulation and Delivery - Chemical - Formulation
Category: Poster Abstract
(M1130-04-22) Anticancer Activity and Pulmonary Delivery of an Antimicrobial Peptide, Nisin ZP, for Non-Small Cell Lung Cancer (NSCLC) Treatment
Monday, October 17, 2022
11:30 AM – 12:30 PM ET
- SP
Suyash M. Patil
St. John's University
Queens, New York, United States - SP
Suyash M. Patil
St. John's University
Queens, New York, United States
Presenting Author(s)
Main Author(s)
Purpose: Lung cancer is the leading cause of cancer deaths globally, with most of the reported cases ( >85%) associated with non-small cell lung cancer (NSCLC). The available chemotherapy and immunotherapy options often lack tumor-targeting leading to systemic toxicity and eventual development of drug resistance, limiting the choice of cancer therapies. Antimicrobial peptides (AMPs) have gained interest as anticancer agents as they selectively target cancer cells and decrease the possibility of resistance. Nisin ZP, an antimicrobial peptide (AMP) produced by the bacterium Lactococcus lactis, has demonstrated anticancer activity in preclinical reports. In this study, we evaluated the anticancer potential and underlying mechanisms of nisin ZP in NSCLC cells. Also, the delivery of peptides by conventional route (oral and parenteral) results in low drug concentration in the lungs where tumor burden is high. Hence, we formulated a nisin ZP dry powder using spray-dryer (NZSD) to facilitate inhaled delivery and eventual high drug concentration at the target site.
Methods: The in vitro cytotoxicity was evaluated using MTT assay in NSCLC (A549) and healthy (HEK293) cells. In addition, kit-based apoptosis, cell cycle, mitochondrial membrane potential, and reactive oxygen species (ROS) assays were performed to determine the mechanism of action. The wound healing, clonogenic, and spheroid assays (volume analysis and spheroid viability) were conducted to support the application of nisin ZP in NSCLC. Nisin ZP (30% w/w) was spray-dried with mannitol, L-leucine, and trehalose in an optimized ratio (75:15:10) using Büchi mini spray-dryer B-290. The spray-dried powder was characterized for yield, peptide content, residual moisture content, solid-state characteristics (Differential Scanning Calorimetry; DSC and Powder X-Ray Diffraction; PXRD) and morphology (Scanning Electron Microscopy; SEM). Aerosolization performance of dry powders was evaluated using Plastiape RS01 dry powder inhaler device in Next Generation Impactor (NGI™️). The stability studies of the dry powder were performed after storage at refrigerated (4 ºC) and room temperature (25 ºC).
Results: Nisin ZP induced selective toxicity in cancer (A549; IC50 132.4 ± 6.4 µM) cells compared to healthy (HEK293; IC50 >300 µM) cells after 48 h of treatment (fig. 1a). Nisin ZP exposure to A549 cells activated the apoptotic pathway and arrested cell cycle progression in the initial G0/G1 growth phase (fig. 1b & c). The cancer cell proliferation was inhibited via non-membranolytic pathways by mitochondrial membrane depolarization (reduction in potential; fig. 1d) and elevation in intracellular ROS levels in a dose-dependent manner (fig. 1e). The clonal expansion (reduction in colony formation; fig. 2a) and wound healing (restricted wound closure; fig 2b) of cancer cells were inhibited by nisin ZP treatment suggesting potential application against highly metastatic NSCLC. In 3D spheroid assay, the multi-dose regimen of nisin ZP at 250 µM (0.071 ± 0.005 mm3) limited the tumor growth compared to control (0.116 ± 0.009 mm3) and 125 µM (0.113 ± 0.007 mm3) treatment group after 12 days (fig. 2d & e), suggesting excellent antitumor potential at twice inhibitory concentration compared to cell monolayer-based assays. This result was further supported by spheroid cell viability, demonstrating significant inhibition in viability for nisin ZP (250 µM) treatment group (fig. 2c).
NZSD formulation revealed a good powder yield of 62.0 ± 4.2% w/w, high peptide content of 326.6 ± 16.3 µg/mg, and low residual moisture content ( < 3.0% w/w; fig. 3a). The presence of an endothermic peak in DSC thermograms (fig. 3b) and attenuated crystalline peaks in PXRD diffractograms (fig. 3c) confirmed the semi-crystalline powder nature with smooth spherical morphology. Aerosolization studies performed using NGI indicated an aerodynamic diameter of 1.12 ± 0.11 μm within the desired range (1-5 µm) and a high fine particle fraction of 92.19 ± 0.71% (fig. 3d), suggesting deposition of powder in respiratory airways of the lung. The anticancer activity of nisin ZP was maintained after fabricating it into NZSD powder and showed a similar inhibitory concentration (fig. 3e) to nisin ZP. Dry powder was stable at both the storage conditions (refrigerated and room temperature) with 95% drug content and semi-crystalline nature, as confirmed by DSC and PXRD studies (data not shown) for three months.
Conclusion: Nisin ZP inhibited lung cancer cell proliferation via the non-membranolytic pathway and exhibited excellent anticancer efficacy against advanced NSCLC in vitro. Further, spray-dried nisin ZP formulation resulted in free-flowing dry powder, enhanced physical stability, and enabled inhaled delivery.
Acknowledgements:This work was funded by the American Association of Colleges of Pharmacy (AACP) via New Investigator Award to Nitesh K. Kunda.

Figure 1. Cytotoxicity and Mechanistic Studies. a. Cytotoxicity (MTT assay) results following Nisin ZP treatment for 48 h in healthy (HEK293) and cancer (A549) Cells. b. Cell cycle arrest of NSCLC cells in G0/G1 phase after nisin ZP treatment in A549 cells. c. Nisin ZP induces apoptosis in NSCLC cells after 24 and 48 h treatment. d. The mitochondrial membrane's polarization is indicated by a decrease in ΔΨM of NSCLC (A549) cells. e. Elevation in intracellular ROS levels following nisin ZP treatment.

Figure 2. Migration and 3D Spheroid Assay. a. Nisin ZP inhibits the colony formation in NSCLC cells, reported as percent growth in colonies. b. Nisin ZP inhibits the metastatic activity of NSCLC cells, shown as % wound healing over time. c. Cell viability assay of 3D spheroids suggesting significant inhibition. d. Spheroid volume growth curve of A549 cells after multiple doses of nisin ZP. e. Representative microscopic images of spheroids.

Figures 3. Spray-dried Nisin ZP formulation development. a. Formulation characteristics of NZSD dry powder. Solid-state characterization of powders - b. Differential Scanning Calorimetry (DSC) Thermograms c. Powder X-Ray Diffraction Studies suggesting semi-crystalline nature. d. Aerosolization performance of NZSD analyzed by drug deposition on various stages of Next Generation Impactor (NGI). e. Cytotoxicity studies (MTT Assay) of nisin ZP and NZSD demonstrated similar anticancer activity.
