Back
Purpose: HCPs constitute a major part of process-related impurities during live virus vaccine (LVV) including COVID-19 vaccine manufacturing using Vero cells. Due to their potential impact to patient safety and product efficacy, residual HCPs in LVVs are often monitored by a commercially available ELISA kit. Recent commercial supply changes of the kit from 1st generation (1G, Catalog # F500) to 2nd generation (2G, Catalog #F975) require a bridging study to understand the differences in HCP values measured from the two kits. The purpose of this study is to compare the two generations of kits in terms of HCP measurement in various intermediate process steps samples and Bulk Drug Substance (BDS) for two different LVV vaccine products to support the assay switch over from 1G kit to 2G kit.
Methods: Commercially available 1st Generation (F500) and 2nd Generation (F975) Vero Cell HCP ELISA kits from Cygnus Technologies were used for side-by-side comparison by testing both F500 kit and F975 kit standard series using the pre-coated microtiter wells and detection antibody of F500 kit, and vice versa, using the pre-coated microtiter wells and detection antibody of F975 kit. Meanwhile, a comparison of kit standard with an in-house prepared standard was also performed. An investigation on the coverage of Vero HCPs for different process intermediates by the respective antibody reagents used in the two kits was performed using 1D/2D SDS-PAGE and 1D/2D-western blots. To understand performance of assay, robustness study was performed by changing incubation time for HRP conjugated antibodies and TMB incubation time.
Results: Our data indicate minimal differences between F500 kit and F975 kit standard in their immunoreactivity to the F500 kit antibody at concentrations ≤75 ng/mL and slight differences at concentrations above 100 ng/mL (Fig. 1), while more differences were seen in the immunoreactivity of the two standards towards F975 kit antibody (Fig. 2), with F975 kit standard showing much stronger binding affinity to the F975 kit antibody than the F500 kit standard. When comparing the values of HCP measured by the two kits, F975 kits detects more HCPs in more purified process intermediates and BDS but less HCPs in HV and CH as compared to F500 kits in LVV1 (Table 1). In LVV2, F975 kit detects less HCPs than F500 kits in all process intermediates, which did not undergo any column purification steps (Table 2). Western blot show that F975 kit antibody has better detection to the HCPs enriched in the ultrafiltered final retentate (UFFR) while the F500 kit preferentially detects slightly more proteins bands in the HV and CH in LVV1.
Conclusion:
Conclusions: ELISA results comparison and 1D/2D Western blots results indicate differences in antibody composition between 1G and 2G Cygnus Vero Cell HCP ELISA kit and high variability of HCP content in the virus harvest of different live virus vaccines (over 10 times difference between HV of LVV1 and LVV2). Bridging data is critical to understand the differences of the two kits and supports the switch over from 1G kit to 2G kit with a clear interpretation on the differences of batch HCP data before and after the switch during methods life cycle management.
.jpg)
Figure 1 Immunoreactivity of F500 and F975 kit standards to the F500 kit antibody
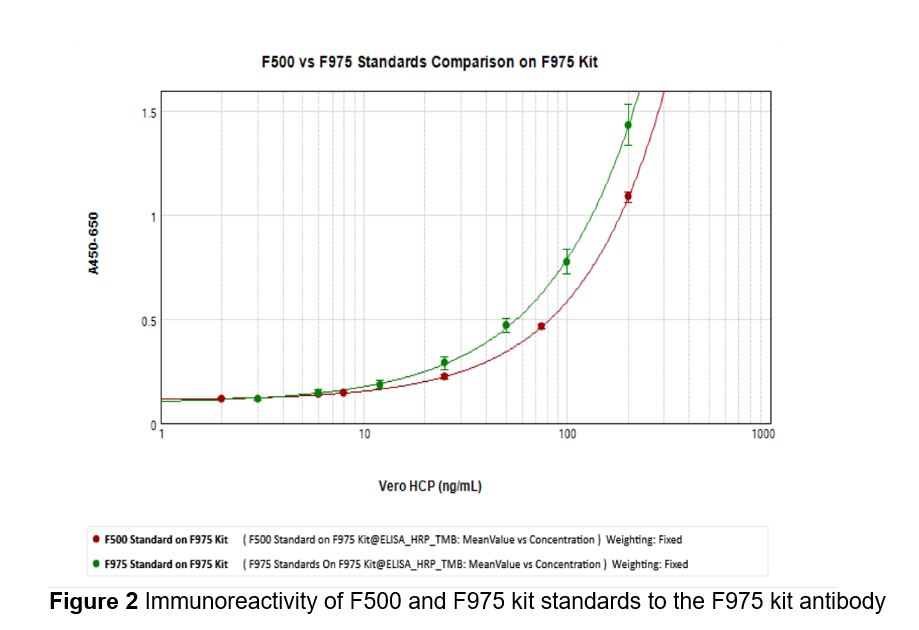
Figure 2 Immunoreactivity of F500 and F975 kit standards to the F975 kit antibody
.jpg)
Table 1 Comparison of HCP values measured by F500 and F975 kit in LVV1 process intermediates and BDS.
Table 2 Comparison of HCP values measured by F500 and F975 kit in LVV2 process intermediates and BRFP.
Bioanalytics - Biomolecular - Vaccines
Category: Poster Abstract
(M1130-09-54) Bridging Two Generations of Commercial ELISA Kits for Residual Vero Cell Host Cell Protein (HCP) Control in Live Virus Vaccines
Monday, October 17, 2022
11:30 AM – 12:30 PM ET

Shruti Patel, MS
Scientist
Merck & Co., Inc.
Kenilworth, New Jersey, United States
Shruti Patel, MS
Scientist
Merck & Co., Inc.
Kenilworth, New Jersey, United States
Presenting Author(s)
Main Author(s)
Purpose: HCPs constitute a major part of process-related impurities during live virus vaccine (LVV) including COVID-19 vaccine manufacturing using Vero cells. Due to their potential impact to patient safety and product efficacy, residual HCPs in LVVs are often monitored by a commercially available ELISA kit. Recent commercial supply changes of the kit from 1st generation (1G, Catalog # F500) to 2nd generation (2G, Catalog #F975) require a bridging study to understand the differences in HCP values measured from the two kits. The purpose of this study is to compare the two generations of kits in terms of HCP measurement in various intermediate process steps samples and Bulk Drug Substance (BDS) for two different LVV vaccine products to support the assay switch over from 1G kit to 2G kit.
Methods: Commercially available 1st Generation (F500) and 2nd Generation (F975) Vero Cell HCP ELISA kits from Cygnus Technologies were used for side-by-side comparison by testing both F500 kit and F975 kit standard series using the pre-coated microtiter wells and detection antibody of F500 kit, and vice versa, using the pre-coated microtiter wells and detection antibody of F975 kit. Meanwhile, a comparison of kit standard with an in-house prepared standard was also performed. An investigation on the coverage of Vero HCPs for different process intermediates by the respective antibody reagents used in the two kits was performed using 1D/2D SDS-PAGE and 1D/2D-western blots. To understand performance of assay, robustness study was performed by changing incubation time for HRP conjugated antibodies and TMB incubation time.
Results: Our data indicate minimal differences between F500 kit and F975 kit standard in their immunoreactivity to the F500 kit antibody at concentrations ≤75 ng/mL and slight differences at concentrations above 100 ng/mL (Fig. 1), while more differences were seen in the immunoreactivity of the two standards towards F975 kit antibody (Fig. 2), with F975 kit standard showing much stronger binding affinity to the F975 kit antibody than the F500 kit standard. When comparing the values of HCP measured by the two kits, F975 kits detects more HCPs in more purified process intermediates and BDS but less HCPs in HV and CH as compared to F500 kits in LVV1 (Table 1). In LVV2, F975 kit detects less HCPs than F500 kits in all process intermediates, which did not undergo any column purification steps (Table 2). Western blot show that F975 kit antibody has better detection to the HCPs enriched in the ultrafiltered final retentate (UFFR) while the F500 kit preferentially detects slightly more proteins bands in the HV and CH in LVV1.
Conclusion:
Conclusions: ELISA results comparison and 1D/2D Western blots results indicate differences in antibody composition between 1G and 2G Cygnus Vero Cell HCP ELISA kit and high variability of HCP content in the virus harvest of different live virus vaccines (over 10 times difference between HV of LVV1 and LVV2). Bridging data is critical to understand the differences of the two kits and supports the switch over from 1G kit to 2G kit with a clear interpretation on the differences of batch HCP data before and after the switch during methods life cycle management.
.jpg)
Figure 1 Immunoreactivity of F500 and F975 kit standards to the F500 kit antibody

Figure 2 Immunoreactivity of F500 and F975 kit standards to the F975 kit antibody
.jpg)
Table 1 Comparison of HCP values measured by F500 and F975 kit in LVV1 process intermediates and BDS.
Table 2 Comparison of HCP values measured by F500 and F975 kit in LVV2 process intermediates and BRFP.
