Back
Purpose: We have identified Nw-hydroxy L-Arginine (NOHA) as a highly sensitive and specific blood-based biomarker to distinguish estrogen receptor negative from estrogen receptor positive (ie, levels <4nM indicative of ER negative disease) in breast cancer patients (U.S. Utility Patent 10,073,099) [1]. In this study we examine the clinical utility of NOHA to monitor neoadjuvant therapy response among triple-negative breast cancer (TNBC) patients
Methods: Participants include newly diagnosed TNBC patients scheduled to undergo neoadjuvant systemic therapy. 3 ml of whole blood were collected at five specific time points of i) pre-chemotherapy, ii) after the second cycle of chemotherapy, iii) after the fourth cycle of chemotherapy, iv) after the last cycle of chemotherapy (i.e., pre-surgery), and v) following surgery. Collected whole blood were processed for plasma isolation, and assessed for NOHA by competitive ELISA method, utilizing a proprietary monoclonal antibody. The ELISA assay results were compared with custom LC-MS NOHA measurement for experimental validation. Statistical difference was set at p< 0.01, with 2 repetitive samples for each tested criteria/condition
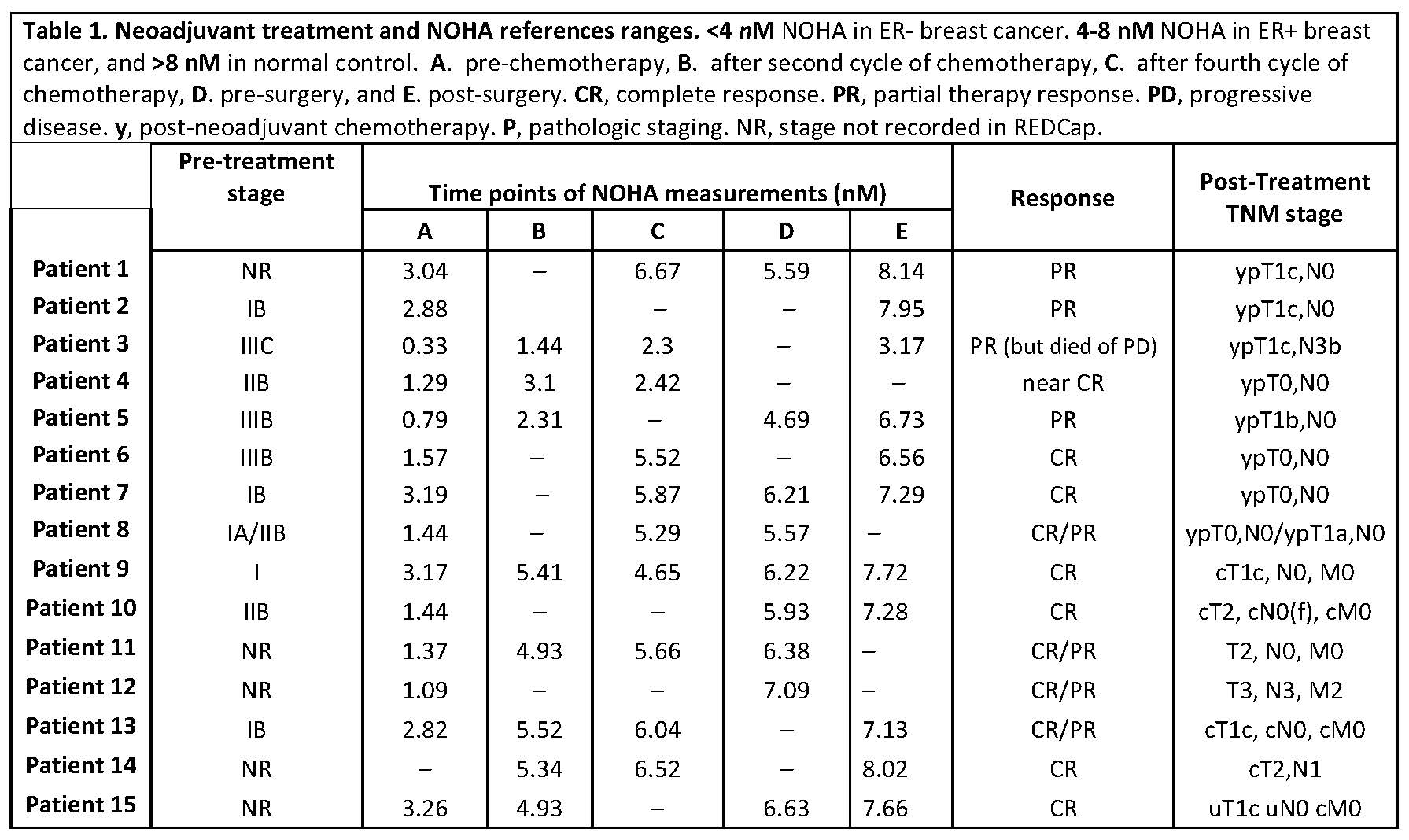
Results: Plasma NOHA levels above 4nM were considered indicative of treatment effectiveness. NOHA levels among all enrolled patients showed a robust correlation with their initial disease burden and was sensitive in predicting clinical and pathologic responses to neoadjuvant treatment (Table 1). Additionally, the sensitivity in NOHA quantification by ELISA assay was comparable with their measurements by LC-MS [2] (data not shown here, but will be presented in poster).
Conclusion: This study provides the first evidence suggesting the utility of NOHA in monitoring clinical and pathologic responses to neoadjuvant therapy. It provides the foundational knowledge for future delineation of NOHA to guide surveillance for/management of metastatic TNBC. It also suggests the utility of the competitive ELISA assay for NOHA measurement, without the need for expensive analytical equipment (such as LC-MS), large lab space, or specialized technical training.
References: [1]. Mohan, S*#., Greenstein, I., Ng, C., Frazier, K., Nguyen, G., Harding, L. (2017). Assessing Nw-hydroxy-L-arginine applicability as a novel ethnic specific estrogen-negative breast cancer marker. Amino Acids Journal. 50(3–4: 373-382. doi: 10.1007/s00726-017-2523-1.
[2]. Mohan, S*., Lawton, S., Palmer, C., Rojas, A.C. (2019). Competitive ELISA method for novel estrogen-negative breast cancer biomarker quantification. Journal of Immunological Methods. 474:112671. doi: 10.1016/j.jim.2019.112671
Acknowledgements:This project is supported in part by Maine Cancer Foundation and University of New England Seed funds
Bioanalytics - Biomolecular - Biomarker Quantification
Category: Poster Abstract
(M1130-09-52) NOHA: A Novel Biomarker to Monitor Treatment Outcome in Triple-Negative Breast Cancer Patients
Monday, October 17, 2022
11:30 AM – 12:30 PM ET
- SM
Srinidi Mohan, Ph.D.
University of New England
Portland, Maine, United States - SM
Srinidi Mohan, Ph.D.
University of New England
Portland, Maine, United States
Presenting Author(s)
Main Author(s)
Purpose: We have identified Nw-hydroxy L-Arginine (NOHA) as a highly sensitive and specific blood-based biomarker to distinguish estrogen receptor negative from estrogen receptor positive (ie, levels <4nM indicative of ER negative disease) in breast cancer patients (U.S. Utility Patent 10,073,099) [1]. In this study we examine the clinical utility of NOHA to monitor neoadjuvant therapy response among triple-negative breast cancer (TNBC) patients
Methods: Participants include newly diagnosed TNBC patients scheduled to undergo neoadjuvant systemic therapy. 3 ml of whole blood were collected at five specific time points of i) pre-chemotherapy, ii) after the second cycle of chemotherapy, iii) after the fourth cycle of chemotherapy, iv) after the last cycle of chemotherapy (i.e., pre-surgery), and v) following surgery. Collected whole blood were processed for plasma isolation, and assessed for NOHA by competitive ELISA method, utilizing a proprietary monoclonal antibody. The ELISA assay results were compared with custom LC-MS NOHA measurement for experimental validation. Statistical difference was set at p< 0.01, with 2 repetitive samples for each tested criteria/condition
Results: Plasma NOHA levels above 4nM were considered indicative of treatment effectiveness. NOHA levels among all enrolled patients showed a robust correlation with their initial disease burden and was sensitive in predicting clinical and pathologic responses to neoadjuvant treatment (Table 1). Additionally, the sensitivity in NOHA quantification by ELISA assay was comparable with their measurements by LC-MS [2] (data not shown here, but will be presented in poster).
Conclusion: This study provides the first evidence suggesting the utility of NOHA in monitoring clinical and pathologic responses to neoadjuvant therapy. It provides the foundational knowledge for future delineation of NOHA to guide surveillance for/management of metastatic TNBC. It also suggests the utility of the competitive ELISA assay for NOHA measurement, without the need for expensive analytical equipment (such as LC-MS), large lab space, or specialized technical training.
References: [1]. Mohan, S*#., Greenstein, I., Ng, C., Frazier, K., Nguyen, G., Harding, L. (2017). Assessing Nw-hydroxy-L-arginine applicability as a novel ethnic specific estrogen-negative breast cancer marker. Amino Acids Journal. 50(3–4: 373-382. doi: 10.1007/s00726-017-2523-1.
[2]. Mohan, S*., Lawton, S., Palmer, C., Rojas, A.C. (2019). Competitive ELISA method for novel estrogen-negative breast cancer biomarker quantification. Journal of Immunological Methods. 474:112671. doi: 10.1016/j.jim.2019.112671
Acknowledgements:This project is supported in part by Maine Cancer Foundation and University of New England Seed funds

