Back
Purpose: Lewisite is a highly toxic chemical warfare agent leading to pain, inflammation, blisters, and toxicity. Lack of readily accessible antidotes to such warfare chemical may result in tragic impact globally. Thus, therapeutic strategies that safely and effectively attenuate this damage remain imperative. Skin is an effective barrier to some chemicals; however, it is ineffective against lewisite which readily permeate into skin and systemic circulation. Thus, an early and effective decontamination is crucial to attenuate cutaneous damage. In addition to decontamination, the delivery of chemical antidotes to lewisite toxicity via the transdermal route can be beneficial in treating not only the local effects on skin, but also the systemic toxicity. 4-phenylbutyric acid (4-PBA) (LogP: 2.5) is a chemical chaperone with therapeutic potential to reduce endoplasmic reticulum (ER) stress induced in case of lewisite toxicity. On the other hand, N-acetylcysteine (NAC) (LogP: -0.71) is another potential antidote to lewisite toxicity with anti-oxidant properties. Delivery of both these antidotes via skin would therefore be good strategy to treat lewisite toxicity. We used phenylarsine oxide (PAO) (LogP: 1.67) as a surrogate for lewisite in our studies1. PAO is a strong oxidant tested for its suitability in understanding arsenicals-mediated tissue injury. We developed a novel formulation that can have applications for simultaneously decontaminating chemical warfare agents and deliver the two antidotes (4-PBA and NAC) into and across the skin for attenuating the cutaneous damage caused by arsenicals.
Methods: Development and validation of HPLC methods for the quantification of 4-PBA, NAC, and PAO was conducted. We screened different percentages of various foaming agents, vehicles, and chemical enhancers for the development of the formulation. F30 (Composition: 20% v/v of a 27% sodium lauryl ether sulfate solution, 5% v/v oleic acid, 10% v/v ethanol, 65% v/v propylene glycol) loaded with 4-PBA and NAC (individually as well their combination, 4-PBA + NAC) was further evaluated for its decontamination efficiency and simultaneous delivery of the antidotes in 5 minutes. Further, the same formulation was tested with 0% ethanol and 10% SLES. The drug loading in all tested formulation was 15.96% w/v of 4-PBA and/or NAC. In vitro permeation testing was carried out using vertical static Franz diffusion cells across dermatomed human skin. PAO dissolved in ethanol (100 mg/mL, 100 µL) was added to the donor chamber to simulate PAO exposure on skin. After 30 minutes of PAO exposure, the donor solution was wiped off using cotton swabs, followed by dosing of 100 µL of formulation. This was allowed to stay for 5 minutes to decontaminate as well as simultaneously delivery the antidotes. Amounts of antidotes and PAO in the skin as well as receptor was analyzed at the end of the study duration.
Results: Amount of PAO delivered into human skin from 100 µL of 100 mg/mL PAO in ethanol was 228.57 ± 28.44 µg/cm2. Amount of PAO in skin decontaminated with formulation F30 with 15.96% w/v NAC and 4-PBA individually and then in combination was 12.30 ± 0.84 µg/cm2, 35.12 ± 8.90 µg/cm2, and 38.50 ± 7.44 µg/cm2 respectively indicating a percentage decontamination efficiency of over 94.62%, 84.61%, and 83.15% respectively (Figure 2). The percentage decontamination efficiency decreased (60.94%) when the ethanol component from the formulation was removed. This indicated that ethanol was a crucial component of the formulation. Also, when SLES content was reduced to 10% from 20%, the decontamination efficiency went down to 65.72% indicating that SLES was imperative at 20% as a foaming agent and played role in decontamination. Further, all formulations were tested for antidote delivery in 5 minutes of application. The formulations with 4-PBA delivered 4-PBA into the skin within 5 minutes in addition to successful decontamination. There was no significant difference observed in the amount of 4-PBA delivered from different formulations. On the other hand, NAC was not delivered into and across the skin in 5 minutes due to the hydrophilic nature of NAC. This suggested the need for investigation of the effect of reapplication of the formulation for a higher delivery of both antidotes into and across skin till 24 hours after reapplication.
Conclusion: Thus, foam F30 successfully decontaminated a significant amount of PAO from the skin and also simultaneously delivered 4-PBA within 5 minutes of application. The formulation was thus identified as the lead formulation to be evaluated for further in vitro and for in vivo efficacy against PAO or other war grade arsenicals.
References: Srivastava RK, Li C, Weng Z, Agarwal A, Elmets CA, Afaq F, et al. Defining cutaneous molecular pathobiology of arsenicals using phenylarsine oxide as a prototype. Sci Rep 2016;6:1–15. https://doi.org/10.1038/srep34865.
Acknowledgements: This project was funded by NIH/NIAMS 1U01AR078544-01 titled “Optimization of Novel Molecular Target-based Drugs for Arsenical Skin Injury”.
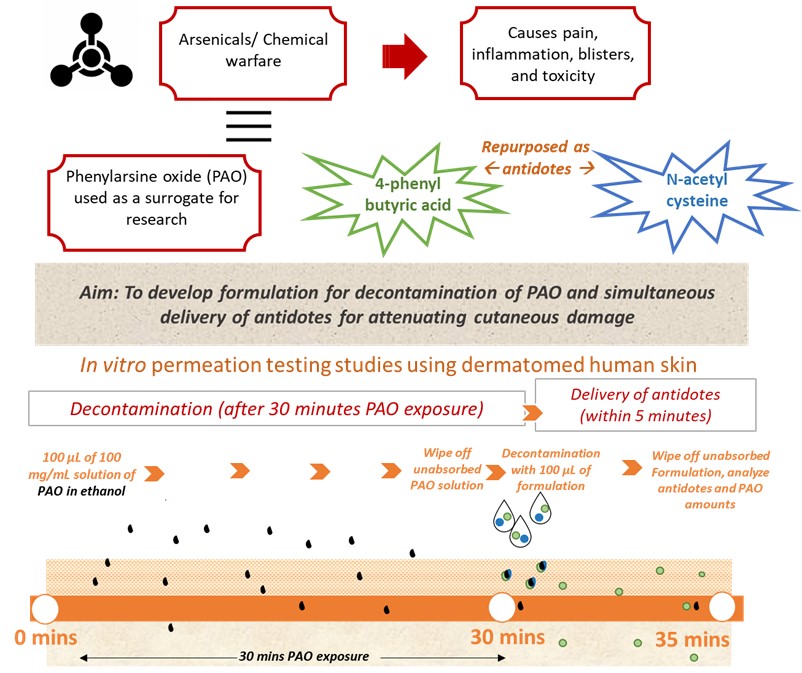
Figure 1: Schematic focusing on our rationale and methodology for testing
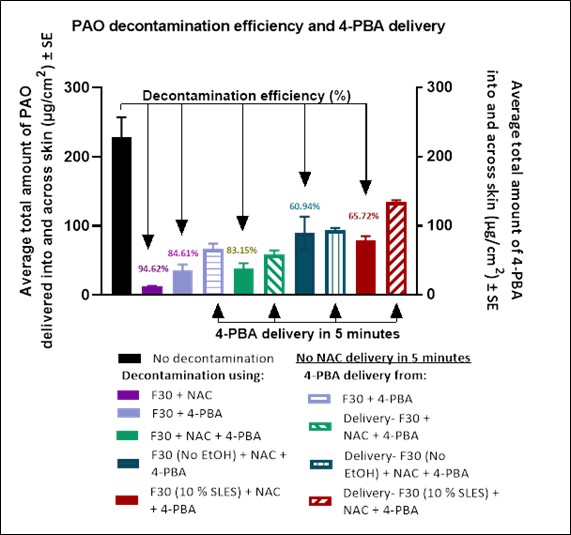
Figure 2: Results for in vitro permeation testing conducted to evaluate decontamination efficiency and simultaneous delivery of antidotes
Formulation and Delivery - Chemical - Drug Delivery
Category: Poster Abstract
(M1130-04-23) Novel Formulation for Simultaneous Decontamination of Chemical Warfare and Delivery of Antidotes for Attenuating Cutaneous Damage Caused by Arsenicals
Monday, October 17, 2022
11:30 PM – 12:30 PM ET

Deepal Vora, MS
PhD candidate
Mercer University
Atlanta, Georgia, United States
Deepal Vora, MS
PhD candidate
Mercer University
Atlanta, Georgia, United States
Presenting Author(s)
Main Author(s)
Purpose: Lewisite is a highly toxic chemical warfare agent leading to pain, inflammation, blisters, and toxicity. Lack of readily accessible antidotes to such warfare chemical may result in tragic impact globally. Thus, therapeutic strategies that safely and effectively attenuate this damage remain imperative. Skin is an effective barrier to some chemicals; however, it is ineffective against lewisite which readily permeate into skin and systemic circulation. Thus, an early and effective decontamination is crucial to attenuate cutaneous damage. In addition to decontamination, the delivery of chemical antidotes to lewisite toxicity via the transdermal route can be beneficial in treating not only the local effects on skin, but also the systemic toxicity. 4-phenylbutyric acid (4-PBA) (LogP: 2.5) is a chemical chaperone with therapeutic potential to reduce endoplasmic reticulum (ER) stress induced in case of lewisite toxicity. On the other hand, N-acetylcysteine (NAC) (LogP: -0.71) is another potential antidote to lewisite toxicity with anti-oxidant properties. Delivery of both these antidotes via skin would therefore be good strategy to treat lewisite toxicity. We used phenylarsine oxide (PAO) (LogP: 1.67) as a surrogate for lewisite in our studies1. PAO is a strong oxidant tested for its suitability in understanding arsenicals-mediated tissue injury. We developed a novel formulation that can have applications for simultaneously decontaminating chemical warfare agents and deliver the two antidotes (4-PBA and NAC) into and across the skin for attenuating the cutaneous damage caused by arsenicals.
Methods: Development and validation of HPLC methods for the quantification of 4-PBA, NAC, and PAO was conducted. We screened different percentages of various foaming agents, vehicles, and chemical enhancers for the development of the formulation. F30 (Composition: 20% v/v of a 27% sodium lauryl ether sulfate solution, 5% v/v oleic acid, 10% v/v ethanol, 65% v/v propylene glycol) loaded with 4-PBA and NAC (individually as well their combination, 4-PBA + NAC) was further evaluated for its decontamination efficiency and simultaneous delivery of the antidotes in 5 minutes. Further, the same formulation was tested with 0% ethanol and 10% SLES. The drug loading in all tested formulation was 15.96% w/v of 4-PBA and/or NAC. In vitro permeation testing was carried out using vertical static Franz diffusion cells across dermatomed human skin. PAO dissolved in ethanol (100 mg/mL, 100 µL) was added to the donor chamber to simulate PAO exposure on skin. After 30 minutes of PAO exposure, the donor solution was wiped off using cotton swabs, followed by dosing of 100 µL of formulation. This was allowed to stay for 5 minutes to decontaminate as well as simultaneously delivery the antidotes. Amounts of antidotes and PAO in the skin as well as receptor was analyzed at the end of the study duration.
Results: Amount of PAO delivered into human skin from 100 µL of 100 mg/mL PAO in ethanol was 228.57 ± 28.44 µg/cm2. Amount of PAO in skin decontaminated with formulation F30 with 15.96% w/v NAC and 4-PBA individually and then in combination was 12.30 ± 0.84 µg/cm2, 35.12 ± 8.90 µg/cm2, and 38.50 ± 7.44 µg/cm2 respectively indicating a percentage decontamination efficiency of over 94.62%, 84.61%, and 83.15% respectively (Figure 2). The percentage decontamination efficiency decreased (60.94%) when the ethanol component from the formulation was removed. This indicated that ethanol was a crucial component of the formulation. Also, when SLES content was reduced to 10% from 20%, the decontamination efficiency went down to 65.72% indicating that SLES was imperative at 20% as a foaming agent and played role in decontamination. Further, all formulations were tested for antidote delivery in 5 minutes of application. The formulations with 4-PBA delivered 4-PBA into the skin within 5 minutes in addition to successful decontamination. There was no significant difference observed in the amount of 4-PBA delivered from different formulations. On the other hand, NAC was not delivered into and across the skin in 5 minutes due to the hydrophilic nature of NAC. This suggested the need for investigation of the effect of reapplication of the formulation for a higher delivery of both antidotes into and across skin till 24 hours after reapplication.
Conclusion: Thus, foam F30 successfully decontaminated a significant amount of PAO from the skin and also simultaneously delivered 4-PBA within 5 minutes of application. The formulation was thus identified as the lead formulation to be evaluated for further in vitro and for in vivo efficacy against PAO or other war grade arsenicals.
References: Srivastava RK, Li C, Weng Z, Agarwal A, Elmets CA, Afaq F, et al. Defining cutaneous molecular pathobiology of arsenicals using phenylarsine oxide as a prototype. Sci Rep 2016;6:1–15. https://doi.org/10.1038/srep34865.
Acknowledgements: This project was funded by NIH/NIAMS 1U01AR078544-01 titled “Optimization of Novel Molecular Target-based Drugs for Arsenical Skin Injury”.

Figure 1: Schematic focusing on our rationale and methodology for testing

Figure 2: Results for in vitro permeation testing conducted to evaluate decontamination efficiency and simultaneous delivery of antidotes
