Back
Industry Encore Posters
JL1012E: Estimating Recurrences Prevented and Costs Avoided with Atezolizumab in Early Non-Small Cell Lung Cancer in the United States
Saturday, October 22, 2022
10:00 AM – 11:00 AM ET

Poster Presenter(s)
OBJECTIVES
To estimate the population-level health and economic benefits associated with atezolizumab as adjuvant treatment for early non-small cell lung cancer (eNSCLC) patients in the US.
METHODS
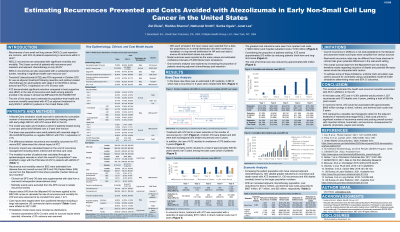
A Monte Carlo simulation was used to estimate the cumulative number of recurrences and deaths prevented, life years saved, and associated costs over a 5-year period by treating eNSCLC patients with atezolizumab versus best supportive care in a given year in the US. Adult patients with resected stage II-IIIA NSCLC receiving adjuvant chemotherapy with PD-L1≥1% were included in the model as the base case population. Model inputs were sourced from US Census data, SEER incidence rates, IMpower010 (NCT02486718) clinical trial data, and other published literature. IMpower010 disease-free survival and overall survival curves were used to extrapolate the number of recurrences and deaths, respectively. Costs included direct, indirect (work productivity), and terminal care costs (last 6 months before death). Confidence intervals (CI) were estimated using 5,000 Monte Carlo simulations.
RESULTS
In any given year, 4,361 eNSCLC patients are in the base case population, of whom 2,558 would experience recurrence within 5 years with previous standard of care. If these 4,361 patients received atezolizumab, an estimated 1,120 patients (95% CI: 1,113–1,127) would avoid recurrence and 397 deaths (95% CI: 389-406) would be prevented, resulting in 779 (95% CI: 763–796) cumulative life years saved. In economic terms, avoidance of recurrences and deaths would reduce future cumulative direct, indirect, and terminal costs by $838 million (95% CI: $832 million - $843 million), $16.3 million (95% CI: $16.1 million - $16.5 million), and $34.5 million (95% CI: $33.8 million - $35.3 million), respectively, over a 5-year time horizon.
CONCLUSIONS
Adjuvant treatment of eNSCLC with atezolizumab is estimated to prevent a significant number of recurrence events and reduce the economic burden associated with recurrence among eNSCLC patients.
To estimate the population-level health and economic benefits associated with atezolizumab as adjuvant treatment for early non-small cell lung cancer (eNSCLC) patients in the US.
METHODS
A Monte Carlo simulation was used to estimate the cumulative number of recurrences and deaths prevented, life years saved, and associated costs over a 5-year period by treating eNSCLC patients with atezolizumab versus best supportive care in a given year in the US. Adult patients with resected stage II-IIIA NSCLC receiving adjuvant chemotherapy with PD-L1≥1% were included in the model as the base case population. Model inputs were sourced from US Census data, SEER incidence rates, IMpower010 (NCT02486718) clinical trial data, and other published literature. IMpower010 disease-free survival and overall survival curves were used to extrapolate the number of recurrences and deaths, respectively. Costs included direct, indirect (work productivity), and terminal care costs (last 6 months before death). Confidence intervals (CI) were estimated using 5,000 Monte Carlo simulations.
RESULTS
In any given year, 4,361 eNSCLC patients are in the base case population, of whom 2,558 would experience recurrence within 5 years with previous standard of care. If these 4,361 patients received atezolizumab, an estimated 1,120 patients (95% CI: 1,113–1,127) would avoid recurrence and 397 deaths (95% CI: 389-406) would be prevented, resulting in 779 (95% CI: 763–796) cumulative life years saved. In economic terms, avoidance of recurrences and deaths would reduce future cumulative direct, indirect, and terminal costs by $838 million (95% CI: $832 million - $843 million), $16.3 million (95% CI: $16.1 million - $16.5 million), and $34.5 million (95% CI: $33.8 million - $35.3 million), respectively, over a 5-year time horizon.
CONCLUSIONS
Adjuvant treatment of eNSCLC with atezolizumab is estimated to prevent a significant number of recurrence events and reduce the economic burden associated with recurrence among eNSCLC patients.


