Back
CME Session
Session: Session 2: Oral Abstract Session
1: Abstract 1: Nivolumab plus ipilimumab plus cabozantinib for previously untreated advanced renal cell carcinoma: results from a discontinued study arm of CheckMate 9ER
Friday, April 22, 2022
09:15 – 09:25 CEST
Location: Level 3, Belle Epoque Ballroom

Bernard Escudier, MD
Institut Gustave-Roussy, France
Presenter(s)
Background: The CheckMate 9ER trial (NCT03141177) originally included a third treatment arm assessing nivolumab plus ipilimumab plus cabozantinib (NIVO+IPI+CABO) for previously untreated advanced renal cell carcinoma (aRCC), which was discontinued early via protocol amendment due to the evolving treatment landscape. Here, we report outcomes for an exploratory cohort of patients randomized to NIVO+IPI+CABO before enrollment discontinuation.
Methods: Patients received NIVO (3 mg/kg) plus IPI (1 mg/kg) Q3W for 4 cycles with once-daily CABO (40 mg), then NIVO (240 mg) Q2W plus once-daily CABO (40 mg). Primary endpoint for CheckMate 9ER was progression-free survival (PFS) by blinded independent central review (BICR); secondary endpoints included overall survival (OS), objective response rate (ORR) by BICR, and safety. Investigator-assessed PFS and ORR were post hoc endpoints for the current analysis.
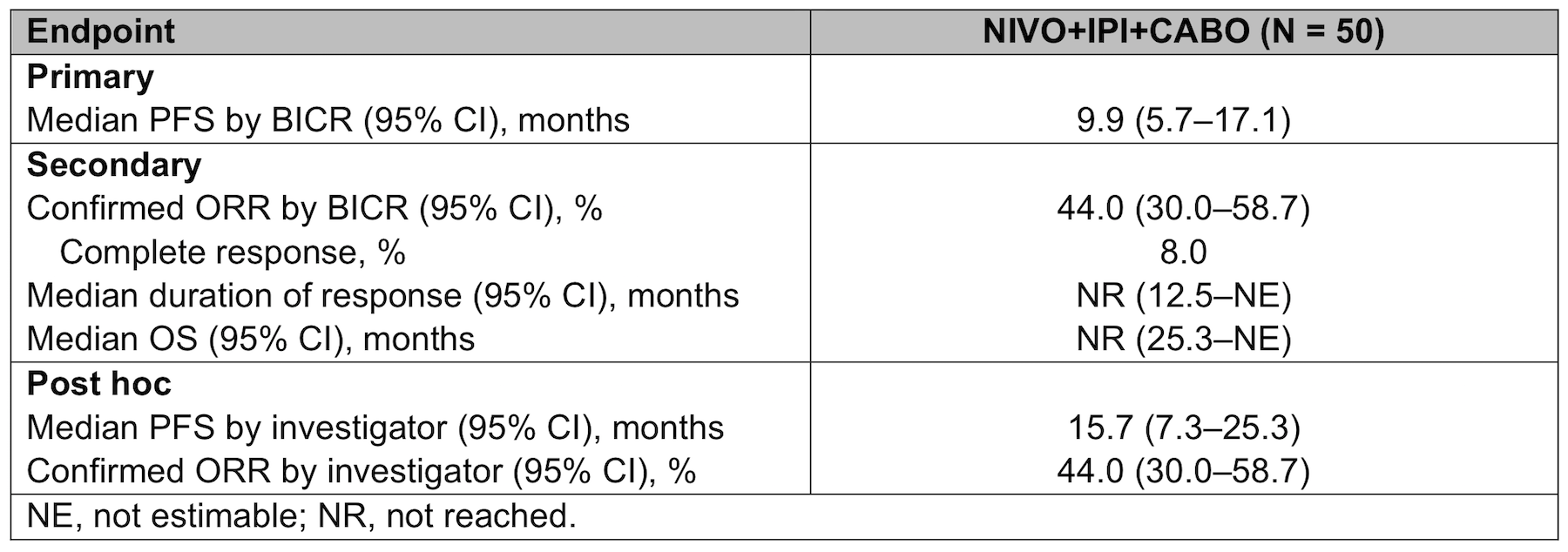
Results: Fifty patients were randomized to receive NIVO+IPI+CABO. Median follow-up (range) for OS was 24.2 (18.6-29.7) months. Efficacy outcomes are summarized in the Table. NIVO, IPI, and CABO dose delays were reported in 78.0%, 38.0%, and 79.6% of patients, respectively, and CABO dose reductions in 63.3%; most dose adjustments were due to adverse events (AEs). Treatment-related AEs occurred in 96.0% of patients; grade 3-4 treatment-related AEs in 80.0%. Treatment-related AEs led to discontinuation of ≥1 treatment in 26.0% of patients (8.0% NIVO and/or IPI only, 6.0% CABO only, 10.0% NIVO+IPI+CABO simultaneously, 2.0% unassigned drug[s]).
Conclusions: This exploratory analysis of a small cohort of patients receiving NIVO+IPI+CABO showed clinical activity in untreated aRCC; however, outcomes may have been impacted by baseline characteristics, as well as the incidence and management of AEs. More definitive evaluation of the triplet regimen for patients with previously untreated aRCC is ongoing in the phase 3 COSMIC-313 trial (NCT03937219).
Methods: Patients received NIVO (3 mg/kg) plus IPI (1 mg/kg) Q3W for 4 cycles with once-daily CABO (40 mg), then NIVO (240 mg) Q2W plus once-daily CABO (40 mg). Primary endpoint for CheckMate 9ER was progression-free survival (PFS) by blinded independent central review (BICR); secondary endpoints included overall survival (OS), objective response rate (ORR) by BICR, and safety. Investigator-assessed PFS and ORR were post hoc endpoints for the current analysis.
Results: Fifty patients were randomized to receive NIVO+IPI+CABO. Median follow-up (range) for OS was 24.2 (18.6-29.7) months. Efficacy outcomes are summarized in the Table. NIVO, IPI, and CABO dose delays were reported in 78.0%, 38.0%, and 79.6% of patients, respectively, and CABO dose reductions in 63.3%; most dose adjustments were due to adverse events (AEs). Treatment-related AEs occurred in 96.0% of patients; grade 3-4 treatment-related AEs in 80.0%. Treatment-related AEs led to discontinuation of ≥1 treatment in 26.0% of patients (8.0% NIVO and/or IPI only, 6.0% CABO only, 10.0% NIVO+IPI+CABO simultaneously, 2.0% unassigned drug[s]).
Conclusions: This exploratory analysis of a small cohort of patients receiving NIVO+IPI+CABO showed clinical activity in untreated aRCC; however, outcomes may have been impacted by baseline characteristics, as well as the incidence and management of AEs. More definitive evaluation of the triplet regimen for patients with previously untreated aRCC is ongoing in the phase 3 COSMIC-313 trial (NCT03937219).

