Back
Regular Abstract
Real-world Evidence
Session: Poster Session
17: Real-world clinical characteristics and treatment patterns in patients with advanced renal cell carcinoma treated with first-line immunotherapy + axitinib in Germany
Location: Poster Hall, Board C5
Amanda Ribbands, BSc; Andrea Leith, BSc; Cameron Forshaw, BSc; Callum Bleasdale, BSc; Mairead Kearney, MB, BCh, MBA, MSc; Claus Fieseler; Karen Rußwurm; Ralf Eckert; Sandra Seseke; Diana Standhaft; Martina Stauch; Monika Schiffer; Jan Dieckhoff; Steffen Baumann; Horste Brenneis; Michael Seidel; Olrik Rau; Silke Schirrmacher-Memmel; Eva Hellmis; Christian Doehn; Carsten Ziske; Andrea Distelrath; Norbert Marschner; Philipp Ivanyi; Carsten Lange; Andreas Janitsky; Martin Bögemann
- GZ
Giovanni Zanotti, PharmD, MSc
Pfizer
New York, New York, United States
Poster Presenter(s)
Background: In 2019, the European Commission approved pembrolizumab+axitinib (P+Ax) and avelumab+axitinib (A+Ax) as first-line (1L) treatment combinations for advanced renal cell carcinoma (aRCC). Little information exists regarding real-world use of these immunotherapy/tyrosine kinase inhibitors (IO/TKIs). We assessed clinical characteristics, treatment patterns and duration in patients with aRCC who received 1L IO/TKI in Germany.
Methods: As part of a point-in-time survey conducted during September-November 2021, recruited physicians completed record forms for their next 4-8 patients with aRCC who received 1L IO/TKI (1:1, P+Ax or A+Ax) and were receiving second-line (2L) treatment at time of data collection. Descriptive analyses were conducted and missing data excluded.
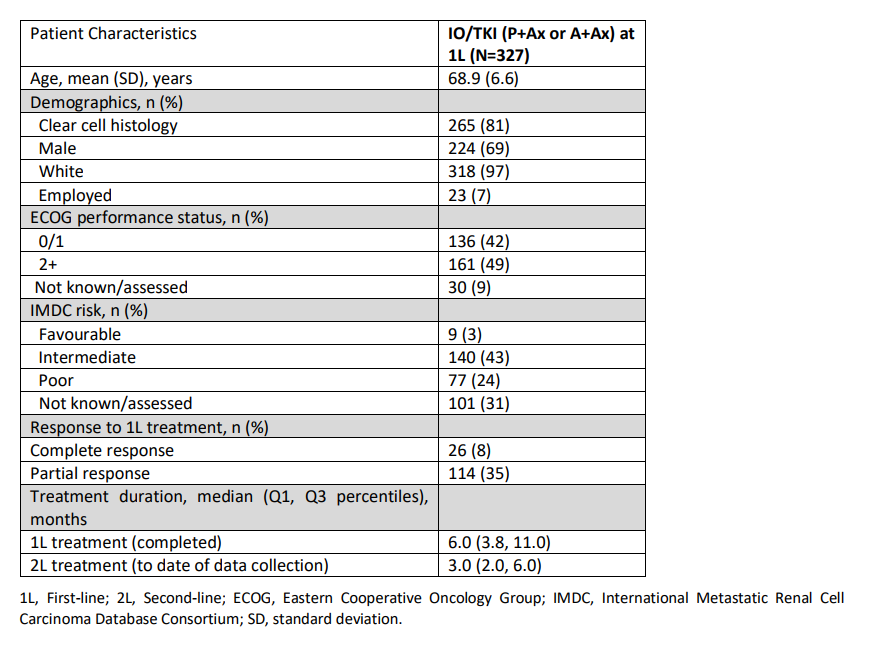
Results: Physicians (n=50) provided data on 327 patients (164 1L P+Ax; 163 1L A+Ax). The current mean patient age was 68.9 years, 69% male, and 67% intermediate/poor IMDC prognostic risk. Median 1L treatment duration was 6.0 months. At 1L, physicians reported that 43% (n=140) of patients had achieved overall objective response (ORR). The most common reasons for stopping 1L therapy were disease progression (46%) and unacceptable tolerability (12%). The most common 2L were nivolumab (35%) and cabozantinib (32%) with an ORR of 32%.
Conclusions: This analysis describes patients who required 2L therapy and their outcomes in the real-world setting in Germany. Patients included in this analysis had a worse prognosis compared with the corresponding phase 3 registrational clinical trials but our findings indicate similar complete response rates with a third of patients responding to 2L therapy. This snapshot of real-world 1L IO/TKI followed by 2L in aRCC demonstrates the benefit of this combination in patients with a worse prognosis. This analysis is limited by excluding patients who remain on 1L IO/TKI without proceeding to 2L therapy and need further analysis.
Methods: As part of a point-in-time survey conducted during September-November 2021, recruited physicians completed record forms for their next 4-8 patients with aRCC who received 1L IO/TKI (1:1, P+Ax or A+Ax) and were receiving second-line (2L) treatment at time of data collection. Descriptive analyses were conducted and missing data excluded.
Results: Physicians (n=50) provided data on 327 patients (164 1L P+Ax; 163 1L A+Ax). The current mean patient age was 68.9 years, 69% male, and 67% intermediate/poor IMDC prognostic risk. Median 1L treatment duration was 6.0 months. At 1L, physicians reported that 43% (n=140) of patients had achieved overall objective response (ORR). The most common reasons for stopping 1L therapy were disease progression (46%) and unacceptable tolerability (12%). The most common 2L were nivolumab (35%) and cabozantinib (32%) with an ORR of 32%.
Conclusions: This analysis describes patients who required 2L therapy and their outcomes in the real-world setting in Germany. Patients included in this analysis had a worse prognosis compared with the corresponding phase 3 registrational clinical trials but our findings indicate similar complete response rates with a third of patients responding to 2L therapy. This snapshot of real-world 1L IO/TKI followed by 2L in aRCC demonstrates the benefit of this combination in patients with a worse prognosis. This analysis is limited by excluding patients who remain on 1L IO/TKI without proceeding to 2L therapy and need further analysis.

