Back
Poster, Podium & Video Sessions
Moderated Poster
MP35: Sexual Function/Dysfunction: Medical, Hormonal & Non-surgical Therapy I
MP35-06: Significant Decrease in Sex Hormone Binding Globulin with Use of Testosterone Undecanoate Capsules (Jatenzo) in Men with Hypogonadism
Saturday, May 14, 2022
4:30 PM – 5:45 PM
Location: Room 222
Alyssa Yee*, Maria Uloko, Irwin Goldstein, San Diego, CA

Alyssa Yee, MD
San Diego Sexual Medicine/Scripps Green Hospital
Poster Presenter(s)
Introduction: Jatenzo (Clarus Therapeutics) which consists of highly lipophilic prodrug, testosterone undecanoate (TU), in a self-emulsifying drug delivery system (SEDDS), is the first FDA-approved (2019) oral testosterone replacement therapy (TRT) for adult males with primary or secondary hypogonadism. The SEDDS allows TU to avoid first pass hepatic metabolism by being absorbed into the enteric lymphatics, then entering the left subclavian vein to be activated to testosterone (T) by esterases. T is subsequently bound to sex hormone binding globulin (SHBG), a hepatic synthesized protein whose level is increased, in part, by hepatic conditions (e.g. fatty liver, non-alcoholic steatohepatitis, cirrhotic liver) and whose level is decreased, in part, by improvement in these hepatic conditions. We wished to study the effect of Jatenzo on SHBG including downstream changes to calculated free T and dihydrotestosterone (DHT) values.
Methods: We reviewed the charts of our first 50 patients using TU capsules. T, SHBG and DHT were measured 7 days after the patient started his optimum dose, defined as the value of TU that resulted in a mid-range T (425-970 ng/dl).
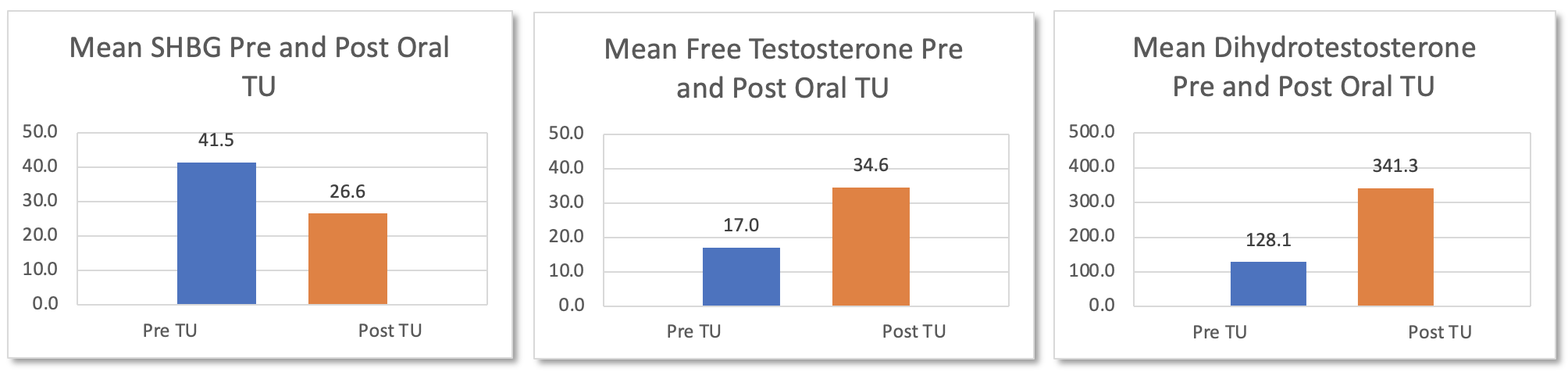
Results: The mean age of our study group was 60.2 ± 9 years. 75% of patients were on TRT previously. The mean testosterone value for these patients prior to TU capsule use was 486 ng/dl, a value higher than clinical trial data because patients were not required to washout prior to starting TU. SHBG lowered from 41.5 nmol/L at baseline to 26.6 nmol/L post treatment (156% decrease). This resulted in changes in free testosterone values from 17.0 ng/dl at baseline to 34.6 ng/dl post-treatment (204% increase) and DHT values from 12.8 ng/dl at baseline to 34.1 ng/dl post-treatment (267% increase). No increase in hematocrit or blood pressure severe enough to require change in patient treatment were reported.
Conclusions: Our initial experience with TU capsules for the treatment of hypogonadism shows a decrease in SHBG resulting in increases in free T and DHT. In contrast to the hepatotoxic effects of previous methylated oral T products, oral TU (Jatenzo) may decrease SHBG by improving liver health. Further research is needed.
Source of Funding: None
Methods: We reviewed the charts of our first 50 patients using TU capsules. T, SHBG and DHT were measured 7 days after the patient started his optimum dose, defined as the value of TU that resulted in a mid-range T (425-970 ng/dl).
Results: The mean age of our study group was 60.2 ± 9 years. 75% of patients were on TRT previously. The mean testosterone value for these patients prior to TU capsule use was 486 ng/dl, a value higher than clinical trial data because patients were not required to washout prior to starting TU. SHBG lowered from 41.5 nmol/L at baseline to 26.6 nmol/L post treatment (156% decrease). This resulted in changes in free testosterone values from 17.0 ng/dl at baseline to 34.6 ng/dl post-treatment (204% increase) and DHT values from 12.8 ng/dl at baseline to 34.1 ng/dl post-treatment (267% increase). No increase in hematocrit or blood pressure severe enough to require change in patient treatment were reported.
Conclusions: Our initial experience with TU capsules for the treatment of hypogonadism shows a decrease in SHBG resulting in increases in free T and DHT. In contrast to the hepatotoxic effects of previous methylated oral T products, oral TU (Jatenzo) may decrease SHBG by improving liver health. Further research is needed.
Source of Funding: None


.jpg)
.jpg)