Back
Poster, Podium & Video Sessions
Podium
PD32: Infections/Inflammation/Cystic Disease of the Genitourinary Tract: Kidney & Bladder III
PD32-10: Kidney Stone Endotoxin Concentration Correlates with Post-Operative Sepsis Following Percutaneous Nephrolithotomy
Saturday, May 14, 2022
5:00 PM – 5:10 PM
Location: Room 255
David T. Tzou*, Farhan Anwar, Ava C. Wong, David T. Harris, Tucson, AZ, Thomas Chi, San Francisco, CA, Gayatri Vedantam, Tucson, AZ

David T. Tzou, MD
Assistant Professor
Podium Presenter(s)
Introduction: Patients with infected kidney stones are at risk for post-operative sepsis following surgical removal of their calculi. Of all the surgical approaches, percutaneous nephrolithotomy (PCNL) remains associated with the highest risk for experiencing post-operative SIRS (severe inflammatory response syndrome). Accurate prediction of patients at risk for SIRS after surgery would be of clinical value. While previous studies have demonstrated infected kidney stones contain variable levels of endotoxin concentrations, these were limited in their rigor and ability to correlate stone immunobiology with important clinical outcomes. The aim of this study was to quantitate endotoxin levels amongst PCNL patients who experienced and did not experience SIRS.
Methods: Between October 2020 and June 2021, urine & stone specimens from consecutive stone patients were prospectively collected as part of the Registry for Stones of the Kidneys and Ureter (ReSKU) at the University of Arizona. Clinical data including stone analyses and post-operative outcomes have been tracked. Equal weights of stones were uniformly broken using an easy-to-replicate ‘beat-beating’ homogenization approach, allowing for consistent measurement of stone endotoxin using a Pierce™ LAL Chromogenic Endotoxin Quantification Kit (ThermoFisher, Waltham, MA). Endotoxin levels from stones removed during PCNL were compared between patients who experienced and did not experience SIRS. Independent T-test was used to determine statistical significance.
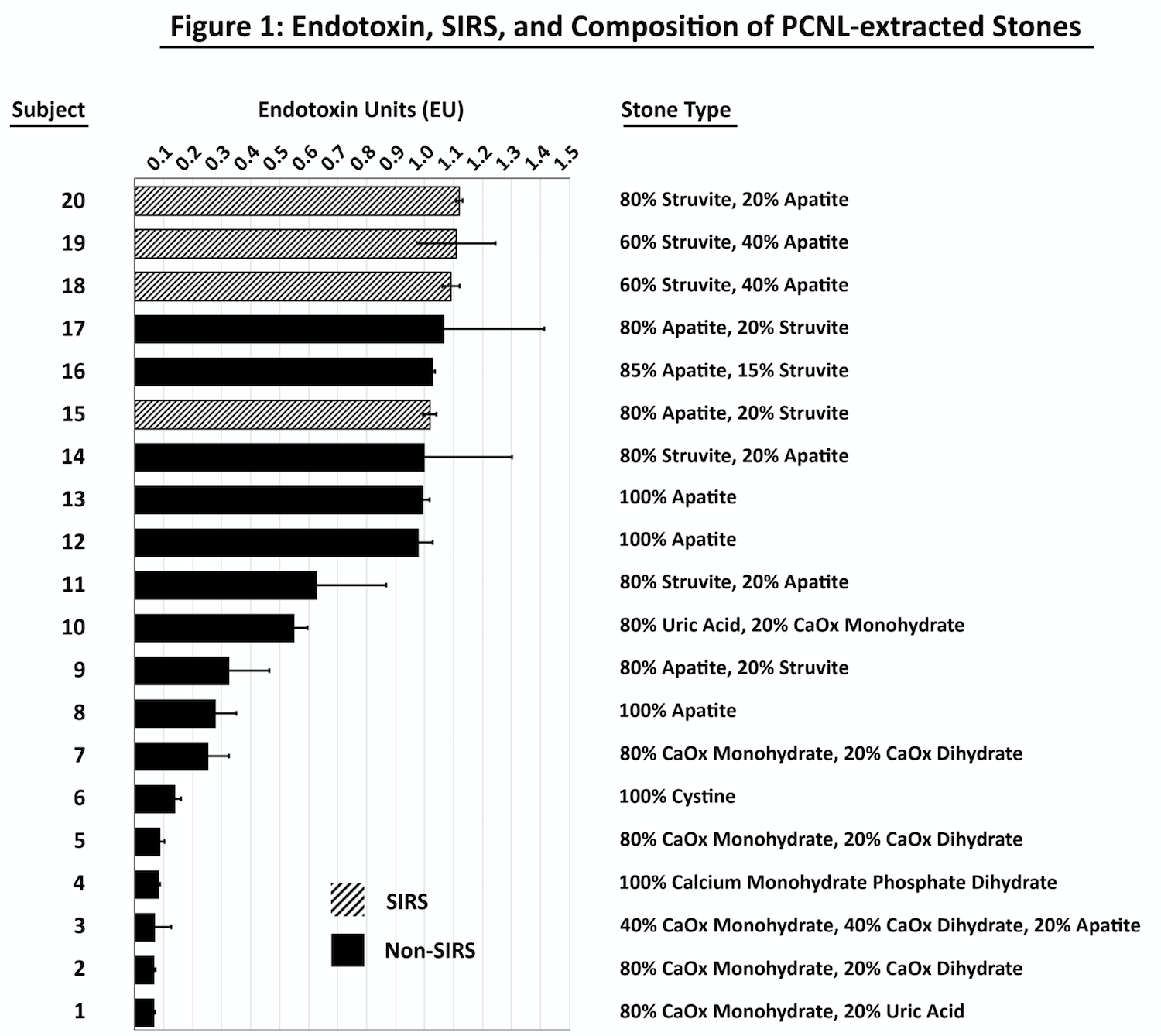
Results: Endotoxin levels amongst 4 patients with infection calculi who experienced SIRS were compared to 16 patients who did not experience SIRS – 8 of which had infection calculi and 8 had non-infection calculi (Figure 1). In the SIRS group, the mean Endotoxin concentration was 1.084 endotoxin units (EU)/ml compared to 0.476 EU/ml in the non-SIRS group (p < 0.01).
Conclusions: Higher kidney stone bacterial endotoxin concentrations are present in patients who experienced SIRS after percutaneous stone removal compared to those who did not. Whether exceeding a threshold endotoxin level corresponds to patients experiencing SIRS warrants future study.
Source of Funding: P30DK081943 – SUBK00015084 - NIH/NIDDK; University of Arizona Health Sciences Career Development Award
Methods: Between October 2020 and June 2021, urine & stone specimens from consecutive stone patients were prospectively collected as part of the Registry for Stones of the Kidneys and Ureter (ReSKU) at the University of Arizona. Clinical data including stone analyses and post-operative outcomes have been tracked. Equal weights of stones were uniformly broken using an easy-to-replicate ‘beat-beating’ homogenization approach, allowing for consistent measurement of stone endotoxin using a Pierce™ LAL Chromogenic Endotoxin Quantification Kit (ThermoFisher, Waltham, MA). Endotoxin levels from stones removed during PCNL were compared between patients who experienced and did not experience SIRS. Independent T-test was used to determine statistical significance.
Results: Endotoxin levels amongst 4 patients with infection calculi who experienced SIRS were compared to 16 patients who did not experience SIRS – 8 of which had infection calculi and 8 had non-infection calculi (Figure 1). In the SIRS group, the mean Endotoxin concentration was 1.084 endotoxin units (EU)/ml compared to 0.476 EU/ml in the non-SIRS group (p < 0.01).
Conclusions: Higher kidney stone bacterial endotoxin concentrations are present in patients who experienced SIRS after percutaneous stone removal compared to those who did not. Whether exceeding a threshold endotoxin level corresponds to patients experiencing SIRS warrants future study.
Source of Funding: P30DK081943 – SUBK00015084 - NIH/NIDDK; University of Arizona Health Sciences Career Development Award


.jpg)
.jpg)