Back
Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0317–0342) SLE – Diagnosis, Manifestations, and Outcomes Poster I: Diagnosis
0326: Mapping Anti-Mitochondrial Antibodies over Time in a Lupus Inception Cohort
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Yann Becker, PhD
Centre de Recherche ARThrite, CHU de Québec - Université Laval
Québec, QC, Canada
Abstract Poster Presenter(s)
Yann Becker1, Éric Boilard1, Emmanuelle Rollet-Labelle1, Christian Lood2, Anne-Sophie Julien3, Joannie Leclerc1, Tania Lévesque1, Murray Urowitz4, John Hanly5, Caroline Gordon6, Sang-Cheol Bae7, Juanita Romero-Diaz8, Jorge Sanchez-Guerrero9, Ann E Clarke10, Sasha Bernatsky11, Daniel Wallace12, David Isenberg13, Anisur Rahman14, Joan Merrill15, Dafna Gladman16, Ian N. Bruce17, Michelle Petri18, Ellen M. Ginzler19, Mary Anne Dooley20, Rosalind Ramsey-Goldman21, Susan Manzi22, Andreas Jönsen23, Graciela Alarcón24, Ronald van Vollenhoven25, Cynthia Aranow26, Guillermo Ruiz-Irastorza27, S. Sam Lim28, Murat Inanc29, Kenneth Kalunian30, Soren Jacobsen31, Christine Peschken32, Diane Kamen33, Anca Askanase34, Jill Buyon35 and Paul R Fortin36, 1Centre de Recherche ARThrite, CHU de Québec - Université Laval, Québec, QC, Canada, 2Division of Rheumatology, University of Washington, Seattle, WA, 3Université Laval, Québec, QC, Canada, 4University of Toronto, University Health Network, Schroeder Arthritis Institute, Toronto, ON, Canada, 5Division of Rheumatology, Queen Elizabeth II Health Sciences Center (Nova Scotia Rehabilitation Site) and Dalhousie University, Halifax, NS, Canada, 6Rheumatology Research Group, Institute of Inflammation and Ageing, College of Medical and Dental Sciences, University of Birmingham, Birmingham, United Kingdom, 7Hanyang University Medical Center, Seoul, Republic of Korea, 8Instituto Nacional de Ciencias Medicas y Nutricion SZ, Ciudad de México, Mexico, 9Mount Sinai Hospital and University Health Network, University of Toronto, Toronto, ON, Canada, 10University of Calgary, Division of Rheumatology, Cumming School of Medicine, Calgary, AB, Canada, 11Research Institute of the McGill University Health Centre, Montréal, QC, Canada, 12Cedars-Sinai Medical Center, Los Angeles, CA, 13University College London, London, United Kingdom, 14Centre for Rheumatology, Department of Medicine, University College London, London, United Kingdom, 15Oklahoma Medical Research Foundation, Oklahoma City, OK, 16Toronto Western Hospital, Schroeder Arthritis Institute, Toronto, ON, Canada, 17Centre for Epidemiology Versus Arthritis, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, United Kingdom, 18Johns Hopkins University School of Medicine, Division of Rheumatology, Baltimore, MD, 19SUNY Downstate Health Sciences University, Department of Medicine, Brooklyn, NY, 20Raleigh Neurology Associates, Chapel Hill, NC, 21Northwestern University Feinberg School of Medicine, Chicago, USA, Chicago, IL, 22Allegheny Health Network, Lupus Center of Excellence, Wexford, PA, 23Department of Clinical Sciences, Lund, Section for Rheumatology, Lund University, Lund and Skåne University Hospital, Lund, Sweden, 24The University of Alabama at Birmingham, Oakland, 25Amsterdam University Medical Centers, Amsterdam, Netherlands, 26Feinstein Institutes for Medical Research, Manhasset, NY, 27Autoimmune Diseases Research Unit, Biocruces Bizkaia Health Research Institute, Hospital Universitario Cruces, UPV/EHU, Barakaldo, Spain, 28Emory University, Atlanta, GA, 29Division of Rheumatology, Department of Internal Medicine, Istanbul Medical Faculty, Istanbul University, Istambul, Turkey, 30University of California San Diego, La Jolla, CA, 31Rigshospitalet, Copenhagen, Denmark, 32University of Manitoba, Winnipeg, MB, Canada, 33Medical University of South Carolina, Charleston, SC, 34Columbia University Medical Center, New York, NY, 35NYU Grossman School of Medicine, New York, NY, 36Centre ARThrite - CHU de Québec - Université Laval, Québec, QC, Canada
Background/Purpose: Mitochondria can be both pro-inflammatory and antigenic. We hypothesize (1) that anti-mitochondrial antibodies (AMA) are present in lupus and (2) can predict outcomes. Our aim was to map three AMAs in the Systemic Lupus International Collaborating Clinics (SLICC) inception cohort and test their effects on clinical outcomes.
Methods: The SLICC inception cohort recruited SLE patients 15 months or less after their diagnosis (1997 ACR classification criteria) from 31 centers in 11 countries. We included all participants with up to 4 biospecimens available as well as healthy controls. We retrieved their sociodemographic and disease characteristic variables [disease activity (SLEDAI-2K), damage (SLICC damage index (SDI)) and medications]. Clinical outcomes of interest were death, arterial (AVE) and venous vascular events; nephritis, SDI, and neuropsychiatric SLE (NPSLE). Covariables included in all models: sex, race/ethnicity, age, time since diagnosis, body mass index (BMI), hypertension, hypercholesterolemia, use of prednisone, antimalarial and immuno-suppressors with the addition of history of past AVE for the AVE models. Antibodies against whole mitochondria (AwMA), mitochondrial DNA (AmtDNA), or RNA (AmtRNA) were assessed using in-house direct ELISAs and reported on a continuous scale. We used Spearman's correlation to evaluate associations between AMAs; Wilcoxon-Mann-Whitney test for correlations between groups; and Cox regressions models using baseline value of each AMA individually as predictor variables adjusted for covariables and multiple imputations for the effects of AMAs on clinical outcomes.
Results: We tested 127 healthy controls and 3450 SLE biospecimens from 1114 SLICC participants and analyzed data from 9788 SLICC clinical visits. There were 89% females with baseline characteristics (mean+sd): age (35.4+13.4 years), SLE duration (0.46+0.35 years), and baseline SLEDAI-2K (5.3+5.3). Correlations between AMAs were low to moderate. Figure 1 shows that the levels of the three AMAs are higher in SLE patients than controls and while AwMA levels clearly increase over time, levels of AmtDNA remain constant and those of AmtRNA show a trend towards increasing over time. Correlations between AMAs and SLEDAI-2K were low (coefficients < 0.20). Multivariable analyses using individual baseline AMA levels as predictor variables adjusted for covariables and with multiple imputations for missing values showed that higher levels of AwMA were predictive of death (HR [95%CI] = 3.03 [1.34, 6.82], p=0.008); lower levels of AmtRNA of AVE (4.54 [1.52, 13.53], p=0.007); and higher levels of both AmtDNA (3.05 [2.05, 4.54], p< 0.0001) and AmtRNA (1.56 [1.12, 2.18], p< 0.008) of nephritis. We did not find associations with any of the three AMAs and venous thrombosis, SDI, or NP-SLE.
Conclusion: Lupus patients have more AMAs than healthy controls and their levels may vary over time. While higher baseline levels of AwMA predicted deaths and higher levels of AmtDNA and AmtRNA predicted nephritis, we found an inverse relationship with lower levels of AmtRNA predicting AVE. These results confirm that AMAs are associated with clinical outcomes in SLE.
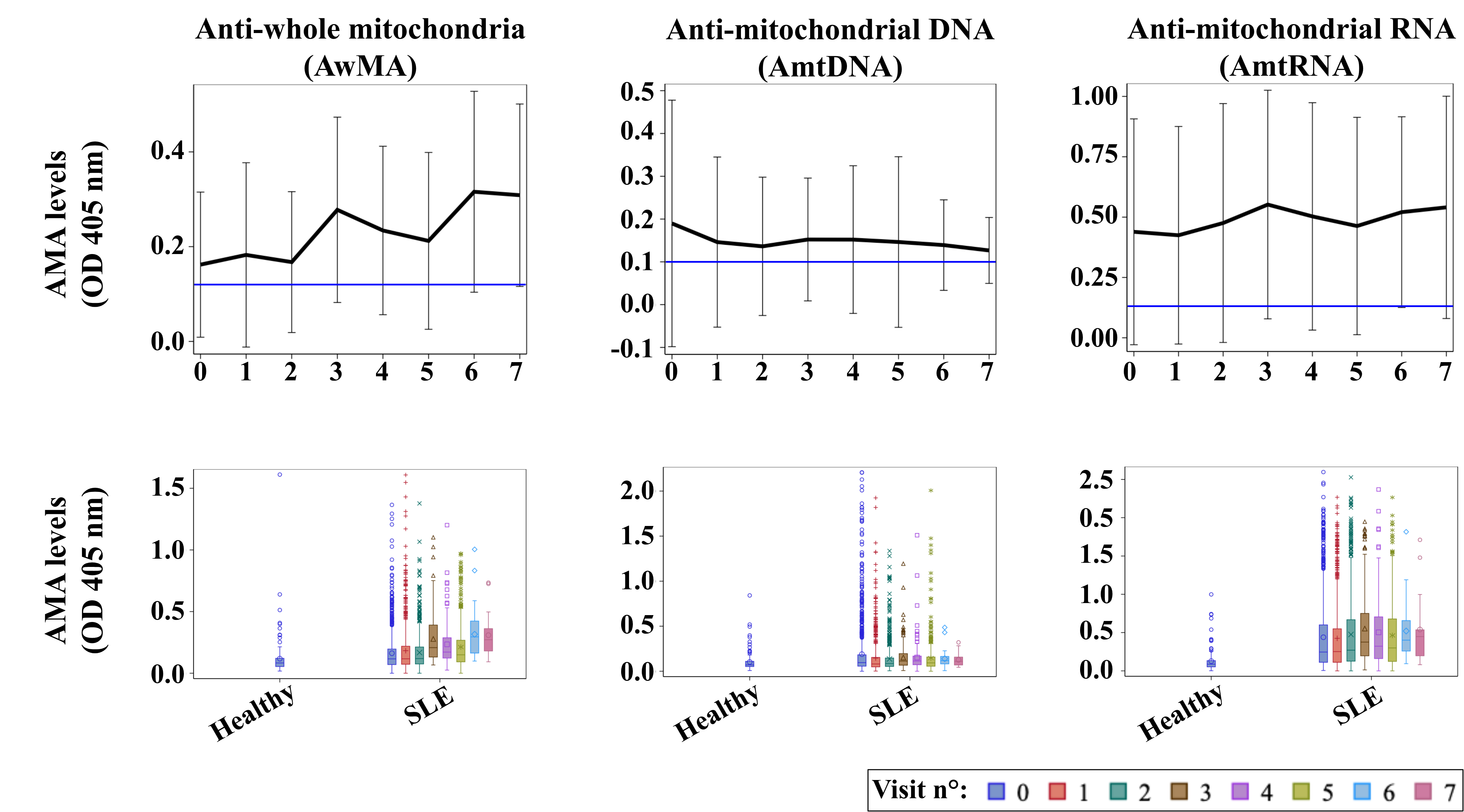 Figure 1: Evolution of anti-mitochondrial antibodies (AMAs) over time.
Figure 1: Evolution of anti-mitochondrial antibodies (AMAs) over time.
The upper row reports on mean optical density values (OD 405nm) of AMAs targeting either whole organelles (AwMA), mitochondrial DNA (AmtDNA), or mitochondrial RNA (AmtRNA). Data are mean ± SD, horizontal blue lines display mean levels of corresponding AMA measured in healthy donors. The lower row shows the distributions of AwMA, AmtDNA and AmtRNA for healthy controls and SLE patients at each visit.
Disclosures: Y. Becker, None; É. Boilard, None; E. Rollet-Labelle, None; C. Lood, Eli Lilly, Gilead Sciences, Pfizer, Bristol-Myers Squibb(BMS), Redd Pharma, Horizon Diagnostic, Exagen Diagnostic; A. Julien, None; J. Leclerc, None; T. Lévesque, None; M. Urowitz, None; J. Hanly, None; C. Gordon, UCB, Amgen, Astra-Zeneca, AbbVie, Sanofi, MGP; S. Bae, None; J. Romero-Diaz, Biogen; J. Sanchez-Guerrero, None; A. Clarke, AstraZeneca, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK); S. Bernatsky, None; D. Wallace, None; D. Isenberg, Merck/MSD, astra zeneca, Eli Lilly, Servier, Amgen; A. Rahman, None; J. Merrill, UCB, GlaxoSmithKline, AbbVie, EMD Serono, Remegen, Celgene/Bristol Myers Squibb, AstraZeneca, Amgen, Janssen, Lilly, Genentech, Aurinia, Astellas, Alexion, Sanofi, Zenas, Proventio; D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; I. Bruce, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, Aurinia, Janssen, GlaxoSmithKlein(GSK); M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee; E. Ginzler, Aurinia Pharma; M. Dooley, None; R. Ramsey-Goldman, None; S. Manzi, AstraZeneca, GlaxoSmithKline (GSK), Exagen Diagnostics Inc, AbbVie, HGS, Cugene, Lilly, UCB Advisory Board, Lupus Foundation of America; A. Jönsen, None; G. Alarcón, None; R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; C. Aranow, None; G. Ruiz-Irastorza, None; S. Lim, None; M. Inanc, None; K. Kalunian, AbbVie/Abbott, Amgen, AstraZeneca, Aurinia, Biogen, Bristol Myers Squibb (BMS), Eli Lilly, Equillium, Genentech, Gilead, Janssen, Roche, Lupus Research Alliance, Pfizer, Sanford Consortium, Viela, Nektar; S. Jacobsen, None; C. Peschken, None; D. Kamen, None; A. Askanase, AstraZeneca, GlaxoSmithKlein(GSK), Aurinia, Amgen, Pfizer, Idorsia, Eli Lilly, UCB, AbbVie/Abbott, Janssen, Bristol-Myers Squibb(BMS); J. Buyon, None; P. Fortin, AstraZeneca, GlaxoSmithKlein(GSK).
Background/Purpose: Mitochondria can be both pro-inflammatory and antigenic. We hypothesize (1) that anti-mitochondrial antibodies (AMA) are present in lupus and (2) can predict outcomes. Our aim was to map three AMAs in the Systemic Lupus International Collaborating Clinics (SLICC) inception cohort and test their effects on clinical outcomes.
Methods: The SLICC inception cohort recruited SLE patients 15 months or less after their diagnosis (1997 ACR classification criteria) from 31 centers in 11 countries. We included all participants with up to 4 biospecimens available as well as healthy controls. We retrieved their sociodemographic and disease characteristic variables [disease activity (SLEDAI-2K), damage (SLICC damage index (SDI)) and medications]. Clinical outcomes of interest were death, arterial (AVE) and venous vascular events; nephritis, SDI, and neuropsychiatric SLE (NPSLE). Covariables included in all models: sex, race/ethnicity, age, time since diagnosis, body mass index (BMI), hypertension, hypercholesterolemia, use of prednisone, antimalarial and immuno-suppressors with the addition of history of past AVE for the AVE models. Antibodies against whole mitochondria (AwMA), mitochondrial DNA (AmtDNA), or RNA (AmtRNA) were assessed using in-house direct ELISAs and reported on a continuous scale. We used Spearman's correlation to evaluate associations between AMAs; Wilcoxon-Mann-Whitney test for correlations between groups; and Cox regressions models using baseline value of each AMA individually as predictor variables adjusted for covariables and multiple imputations for the effects of AMAs on clinical outcomes.
Results: We tested 127 healthy controls and 3450 SLE biospecimens from 1114 SLICC participants and analyzed data from 9788 SLICC clinical visits. There were 89% females with baseline characteristics (mean+sd): age (35.4+13.4 years), SLE duration (0.46+0.35 years), and baseline SLEDAI-2K (5.3+5.3). Correlations between AMAs were low to moderate. Figure 1 shows that the levels of the three AMAs are higher in SLE patients than controls and while AwMA levels clearly increase over time, levels of AmtDNA remain constant and those of AmtRNA show a trend towards increasing over time. Correlations between AMAs and SLEDAI-2K were low (coefficients < 0.20). Multivariable analyses using individual baseline AMA levels as predictor variables adjusted for covariables and with multiple imputations for missing values showed that higher levels of AwMA were predictive of death (HR [95%CI] = 3.03 [1.34, 6.82], p=0.008); lower levels of AmtRNA of AVE (4.54 [1.52, 13.53], p=0.007); and higher levels of both AmtDNA (3.05 [2.05, 4.54], p< 0.0001) and AmtRNA (1.56 [1.12, 2.18], p< 0.008) of nephritis. We did not find associations with any of the three AMAs and venous thrombosis, SDI, or NP-SLE.
Conclusion: Lupus patients have more AMAs than healthy controls and their levels may vary over time. While higher baseline levels of AwMA predicted deaths and higher levels of AmtDNA and AmtRNA predicted nephritis, we found an inverse relationship with lower levels of AmtRNA predicting AVE. These results confirm that AMAs are associated with clinical outcomes in SLE.
 Figure 1: Evolution of anti-mitochondrial antibodies (AMAs) over time.
Figure 1: Evolution of anti-mitochondrial antibodies (AMAs) over time. The upper row reports on mean optical density values (OD 405nm) of AMAs targeting either whole organelles (AwMA), mitochondrial DNA (AmtDNA), or mitochondrial RNA (AmtRNA). Data are mean ± SD, horizontal blue lines display mean levels of corresponding AMA measured in healthy donors. The lower row shows the distributions of AwMA, AmtDNA and AmtRNA for healthy controls and SLE patients at each visit.
Disclosures: Y. Becker, None; É. Boilard, None; E. Rollet-Labelle, None; C. Lood, Eli Lilly, Gilead Sciences, Pfizer, Bristol-Myers Squibb(BMS), Redd Pharma, Horizon Diagnostic, Exagen Diagnostic; A. Julien, None; J. Leclerc, None; T. Lévesque, None; M. Urowitz, None; J. Hanly, None; C. Gordon, UCB, Amgen, Astra-Zeneca, AbbVie, Sanofi, MGP; S. Bae, None; J. Romero-Diaz, Biogen; J. Sanchez-Guerrero, None; A. Clarke, AstraZeneca, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK); S. Bernatsky, None; D. Wallace, None; D. Isenberg, Merck/MSD, astra zeneca, Eli Lilly, Servier, Amgen; A. Rahman, None; J. Merrill, UCB, GlaxoSmithKline, AbbVie, EMD Serono, Remegen, Celgene/Bristol Myers Squibb, AstraZeneca, Amgen, Janssen, Lilly, Genentech, Aurinia, Astellas, Alexion, Sanofi, Zenas, Proventio; D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; I. Bruce, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, Aurinia, Janssen, GlaxoSmithKlein(GSK); M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee; E. Ginzler, Aurinia Pharma; M. Dooley, None; R. Ramsey-Goldman, None; S. Manzi, AstraZeneca, GlaxoSmithKline (GSK), Exagen Diagnostics Inc, AbbVie, HGS, Cugene, Lilly, UCB Advisory Board, Lupus Foundation of America; A. Jönsen, None; G. Alarcón, None; R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; C. Aranow, None; G. Ruiz-Irastorza, None; S. Lim, None; M. Inanc, None; K. Kalunian, AbbVie/Abbott, Amgen, AstraZeneca, Aurinia, Biogen, Bristol Myers Squibb (BMS), Eli Lilly, Equillium, Genentech, Gilead, Janssen, Roche, Lupus Research Alliance, Pfizer, Sanford Consortium, Viela, Nektar; S. Jacobsen, None; C. Peschken, None; D. Kamen, None; A. Askanase, AstraZeneca, GlaxoSmithKlein(GSK), Aurinia, Amgen, Pfizer, Idorsia, Eli Lilly, UCB, AbbVie/Abbott, Janssen, Bristol-Myers Squibb(BMS); J. Buyon, None; P. Fortin, AstraZeneca, GlaxoSmithKlein(GSK).

