Back
Poster Session D
Myopathic rheumatic diseases (polymyositis, dermatomyositis, inclusion body myositis)
Session: (1856–1887) Muscle Biology, Myositis and Myopathies Poster II
1865: Internet-based Enrollment for Myositis Studies Utilizing Patient Self-reported Diagnostic Criteria
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- RL
Raisa Lomanto Silva, MD
University of Pittsburgh Medical Center
Pittsburgh, PA, United States
Abstract Poster Presenter(s)
Raisa Lomanto Silva1, Silvia Martinez Laverde1, Siamak Moghadam-Kia1, Nicole Neiman2, Dana Ascherman3, Chester Oddis3 and Rohit Aggarwal4, 1University of Pittsburgh Medical Center, Pittsburgh, PA, 2Division of Rheumatology and Clinical Immunology, Department of Medicine, Pittsburgh, PA, 3University of Pittsburgh, Pittsburgh, PA, 4Division of Rheumatology and Clinical Immunology, Department of Medicine, University of Pittsburgh, Pittsburgh, PA
Background/Purpose: Recruitment is a major challenge in myositis clinical studies, which is hindered by the rarity and heterogeneity of the disease. Internet-based clinical studies using telemedicine, mobile apps, and wearable devices are a viable solution for enrolling potential subjects. However, establishing criteria to verify the reliability of a patient's self-reported diagnosis of myositis is essential to improve the feasibility and validity of internet-based recruitment. Our aim was to develop a practical and valid criteria for patient-reported diagnosis of myositis for future internet-based studies.
Methods: The study design was prospective and observational with remote recruitment from anywhere in the US. Myositis patients self-recruited using either a mobile app or the study website. Each patient provided their physician-reported diagnosis (dermatomyositis [DM], polymyositis [PM], necrotizing myopathy [NM]) and self-reported relevant disease diagnostic criteria (DM rash, proximal muscle weakness, muscle/skin biopsy consistent with myositis, EMG consistent with myopathy, creatine kinase [CK] elevation, and/or positive myositis autoantibodies [MAAb]). This was followed by a myositis expert (MD) review of medical records to evaluate the accuracy of diagnosis and disease criteria. We evaluated each of these criterion individually and in combination, to determine optimal sensitivity (SN), positive predictive value (PPV), and percentage agreement with kappa statistics for MD confirmed diagnosis.
Results: A total of 121 remotely-screened patients underwent a review of their medical records by an MD. Patients were predominantly female (n=87, 72%) and white (n=103, 85%), with a mean age of 56.1 (SD 13.5). Of the participants, 65 (53%) had a diagnosis of DM, 49 of PM (40%), and 7 of NM (5.7%). Proximal muscle weakness (n=107, 88%) was the most common criterion reported by patients, followed by CK elevation (n=102, 84%), and positive biopsy (n=79, 65%). EMG, DM rash, and MAAb positivity were reported by 60% (n=73), 56% (n=68), and 37% (n=45) of patients, respectively. The individual criteria of DM rash, weakness, EMG, and CK all had ≥94% SN for IIM diagnosis, with a PPV ranging from 89% for EMG to >97% for rash, weakness, and CK elevation. DM rash and weakness had the best agreement (99% and 96.7% respectively) with MD review. In a combined analysis for IIM diagnosis, weakness and CK elevation had 93% SN and 94.6% PPV. For diagnosis of DM, the combination of DM rash and weakness had 95.3% SN and 95% PPV, resulting in 94% agreement with MD diagnosis. For PM/NM, the combinations of proximal muscle weakness and CK elevation or diagnostic EMG had >94% SN and >94% PPV, with 92.8% and 96.4% agreement respectively.
Conclusion: Patient self-reported myositis diagnostic criteria had high SN, PPV, and agreement with exception of MAAb positivity. In this large national study, patients' self-report of physician-diagnosed myositis along with self-reported clinical features had excellent sensitivity for the identification of myositis and its subtypes. These criteria can guide the recruitment of subjects for future internet-based studies.
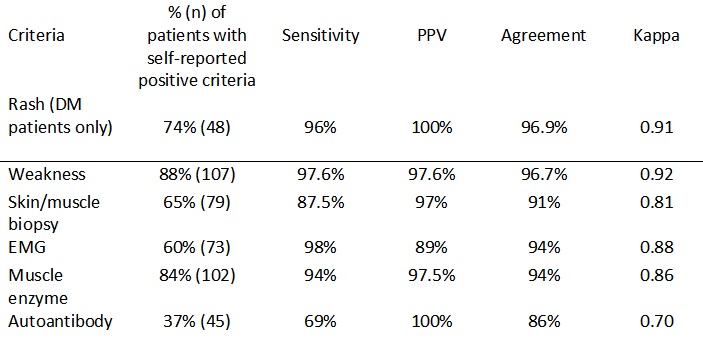 Table 1. Disease criteria for IIM and its' sensitivity, PPV, percentage agreement and, Kappa
Table 1. Disease criteria for IIM and its' sensitivity, PPV, percentage agreement and, Kappa
.jpg) Table 2. Combination of disease criteria and its' sensitivity, PPV, percentage agreement, and Kappa for IIM, DM, and PM/NM
Table 2. Combination of disease criteria and its' sensitivity, PPV, percentage agreement, and Kappa for IIM, DM, and PM/NM
Disclosures: R. Lomanto Silva, None; S. Martinez Laverde, None; S. Moghadam-Kia, None; N. Neiman, None; D. Ascherman, None; C. Oddis, None; R. Aggarwal, Mallinckrodt, Bristol Myers Squibb, EMD Serono, Pfizer, Octapharma, CSL Behring, Q32, Kezar, AstraZeneca, Alexion, Argenx, Boehringer Ingelheim, Corbus, Janssen, Kyverna, Roivant, AbbVie, Jubilant, Orphazyme, Genentech.
Background/Purpose: Recruitment is a major challenge in myositis clinical studies, which is hindered by the rarity and heterogeneity of the disease. Internet-based clinical studies using telemedicine, mobile apps, and wearable devices are a viable solution for enrolling potential subjects. However, establishing criteria to verify the reliability of a patient's self-reported diagnosis of myositis is essential to improve the feasibility and validity of internet-based recruitment. Our aim was to develop a practical and valid criteria for patient-reported diagnosis of myositis for future internet-based studies.
Methods: The study design was prospective and observational with remote recruitment from anywhere in the US. Myositis patients self-recruited using either a mobile app or the study website. Each patient provided their physician-reported diagnosis (dermatomyositis [DM], polymyositis [PM], necrotizing myopathy [NM]) and self-reported relevant disease diagnostic criteria (DM rash, proximal muscle weakness, muscle/skin biopsy consistent with myositis, EMG consistent with myopathy, creatine kinase [CK] elevation, and/or positive myositis autoantibodies [MAAb]). This was followed by a myositis expert (MD) review of medical records to evaluate the accuracy of diagnosis and disease criteria. We evaluated each of these criterion individually and in combination, to determine optimal sensitivity (SN), positive predictive value (PPV), and percentage agreement with kappa statistics for MD confirmed diagnosis.
Results: A total of 121 remotely-screened patients underwent a review of their medical records by an MD. Patients were predominantly female (n=87, 72%) and white (n=103, 85%), with a mean age of 56.1 (SD 13.5). Of the participants, 65 (53%) had a diagnosis of DM, 49 of PM (40%), and 7 of NM (5.7%). Proximal muscle weakness (n=107, 88%) was the most common criterion reported by patients, followed by CK elevation (n=102, 84%), and positive biopsy (n=79, 65%). EMG, DM rash, and MAAb positivity were reported by 60% (n=73), 56% (n=68), and 37% (n=45) of patients, respectively. The individual criteria of DM rash, weakness, EMG, and CK all had ≥94% SN for IIM diagnosis, with a PPV ranging from 89% for EMG to >97% for rash, weakness, and CK elevation. DM rash and weakness had the best agreement (99% and 96.7% respectively) with MD review. In a combined analysis for IIM diagnosis, weakness and CK elevation had 93% SN and 94.6% PPV. For diagnosis of DM, the combination of DM rash and weakness had 95.3% SN and 95% PPV, resulting in 94% agreement with MD diagnosis. For PM/NM, the combinations of proximal muscle weakness and CK elevation or diagnostic EMG had >94% SN and >94% PPV, with 92.8% and 96.4% agreement respectively.
Conclusion: Patient self-reported myositis diagnostic criteria had high SN, PPV, and agreement with exception of MAAb positivity. In this large national study, patients' self-report of physician-diagnosed myositis along with self-reported clinical features had excellent sensitivity for the identification of myositis and its subtypes. These criteria can guide the recruitment of subjects for future internet-based studies.
 Table 1. Disease criteria for IIM and its' sensitivity, PPV, percentage agreement and, Kappa
Table 1. Disease criteria for IIM and its' sensitivity, PPV, percentage agreement and, Kappa.jpg) Table 2. Combination of disease criteria and its' sensitivity, PPV, percentage agreement, and Kappa for IIM, DM, and PM/NM
Table 2. Combination of disease criteria and its' sensitivity, PPV, percentage agreement, and Kappa for IIM, DM, and PM/NM Disclosures: R. Lomanto Silva, None; S. Martinez Laverde, None; S. Moghadam-Kia, None; N. Neiman, None; D. Ascherman, None; C. Oddis, None; R. Aggarwal, Mallinckrodt, Bristol Myers Squibb, EMD Serono, Pfizer, Octapharma, CSL Behring, Q32, Kezar, AstraZeneca, Alexion, Argenx, Boehringer Ingelheim, Corbus, Janssen, Kyverna, Roivant, AbbVie, Jubilant, Orphazyme, Genentech.

