Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0589–0628) RA – Etiology and Pathogenesis Poster
0600: Correlates Between Rheumatoid Arthritis Clinical Factors and Synovial Cell Phenotypes: Data from the Accelerated Medicines Partnership
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall

Katherine Liao, MD, MPH
Brigham and Women's Hospital
Boston, MA, United States
Abstract Poster Presenter(s)
Dana Weisenfeld1, Fan Zhang2, Laura Donlin3, Anna Jonsson1, William Apruzzese1, Debbie Campbell4, Ellen Gravallese5, Larry Moreland6, Susan Goodman3, Michael Brenner5, Soumya Raychaudhuri1, Andrew Filer7, Jennifer Anolik8, Vivian Bykerk3 and Katherine Liao1, 1Brigham and Women's Hospital, Boston, MA, 2University of Colorado, Aurora, CO, 3Hospital for Special Surgery, New York, NY, 4University of Rochester, Rochester, 5Brigham and Women's Hospital, Harvard Medical School, Boston, MA, 6University of Colorado, Denver, CO, 7University of Birmingham, Birmingham, United Kingdom, 8University of Rochester Medical Center, Rochester, NY
Background/Purpose: Prior studies have characterized the diverse cell types in the RA synovium through a multi-center consortium incorporating RNA-seq data. Due to the novelty of this approach, there are limited data on the associations between cell types and states with patient level clinical measurements. Using the largest cohort to date with clinical and multi-cell type data, the objective of this study was to determine associations between RA clinical factors and cell types at the patient level.
Methods: AMP RA recruited patients from 13 centers with inclusion criteria as previously described1. Briefly, subjects were age≥18, fulfilled the 1987 or 2010 ACR/EULAR RA Classification criteria with ≥2 tender and swollen joints and CDAI≥10. Subjects were additionally selected based on treatment status: (i) DMARD naïve, (ii) methotrexate (MTX) inadequate, (iii) TNFi inadequate responders. Demographics, RA treatments at baseline (no DMARD, nbDMARD/no bDMARD, any bDMARD), and RA clinical factors, ESR, CRP, were assessed; CDAI and DAS28-CRP were calculated. Biopsies were performed on an inflamed joint and tissue were processed as previously described to determine synovial cellular phenotype to obtain cell type % and cell type abundance phenotype (CTAP)1. The 6 CTAPs identified named by the dominant cell type were: (1) endothelial, fibroblast, and myeloid cells (EFM), (2) fibroblasts (F), (3) myeloid, (4) T- and B-cells (TB), (5) T cells and fibroblasts (TF), (6) T and myeloid cells (TM. We performed correlations between key RA clinical data (Figure 1) and cell type % using Spearman's correlation for continuous variables and point bi-seral for binary variables. Chi square or ANOVA were performed to test differences in means or proportions, respectively across CTAP categories. In this discovery cohort, we focused on associations p< 0.01, and reported p< 0.05.
Results: We studied n=105 subjects, mean age 56 years, 74% female, 70% White, 15% Black, 31% Hispanic; 85% were seropositive, mean RA duration 6.6 years, DAS28-CRP3 4.8, and hsCRP 18.1mg/L. Anti-CCP and RF positivity correlated with low % myeloid cells, while RF+ was correlated with high % T-cells (Figure 1). Higher DAS28-CRP was correlated with higher % T cells in the synovium. Across CTAPs, the TF cluster had the highest HAQ and patient global scores (Table 2). When examining variation across treatments, subjects on MTX or other nbDMARDs alone had higher %B-cells than other groups. For subjects on bDMARDs, the majority belonged to the EFM CTAP (57%), while none were categorized as TF. No significant difference was observed across CTAPs for age, sex, RA disease duration, DAS28-CRP, and hsCRP.
Conclusion: In this comprehensive screen of clinical factors measured at the time of synovial biopsy, we observed differential associations between cell phenotypes with bDMARD vs nbDMARD use, suggesting that RA therapies may impact cell composition in the synovium. Future directions include longitudinal studies of treatment response to determine whether cell phenotypes can improve our ability to select the optimal RA treatment for each patient.
1Zhang, Jonsson, et al., bioRxiv 2022.02.25.481990
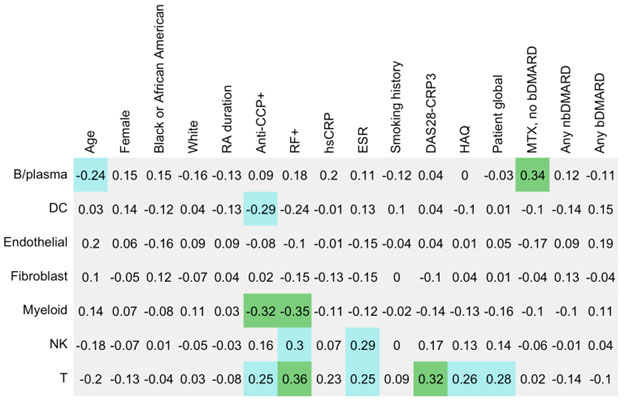 Figure 1. Correlations between cell type % with clinical factors; p-value < 0.01 in green, p≥0.01 and < 0.05 in blue.
Figure 1. Correlations between cell type % with clinical factors; p-value < 0.01 in green, p≥0.01 and < 0.05 in blue.
.jpg)
Disclosures: D. Weisenfeld, None; F. Zhang, None; L. Donlin, None; A. Jonsson, Amgen; W. Apruzzese, None; D. Campbell, None; E. Gravallese, New England Journal of Medicine, Textbook Rheumatology, AMN healthcare, CVS, American Well Corporation, Cerner Corp; L. Moreland, None; S. Goodman, Novartis, UCB; M. Brenner, GSK, 4FO Ventures, Mestag Therapeutics; S. Raychaudhuri, Mestag, Inc, Rheos Medicines, Janssen, Pfizer, Biogen; A. Filer, None; J. Anolik, None; V. Bykerk, Amgen, Bristol-Myers Squibb(BMS), Genzyme, Brainstorm, Gilead, Regeneron, UCB, Pfizer, Sanofi, Aventis; K. Liao, None.
Background/Purpose: Prior studies have characterized the diverse cell types in the RA synovium through a multi-center consortium incorporating RNA-seq data. Due to the novelty of this approach, there are limited data on the associations between cell types and states with patient level clinical measurements. Using the largest cohort to date with clinical and multi-cell type data, the objective of this study was to determine associations between RA clinical factors and cell types at the patient level.
Methods: AMP RA recruited patients from 13 centers with inclusion criteria as previously described1. Briefly, subjects were age≥18, fulfilled the 1987 or 2010 ACR/EULAR RA Classification criteria with ≥2 tender and swollen joints and CDAI≥10. Subjects were additionally selected based on treatment status: (i) DMARD naïve, (ii) methotrexate (MTX) inadequate, (iii) TNFi inadequate responders. Demographics, RA treatments at baseline (no DMARD, nbDMARD/no bDMARD, any bDMARD), and RA clinical factors, ESR, CRP, were assessed; CDAI and DAS28-CRP were calculated. Biopsies were performed on an inflamed joint and tissue were processed as previously described to determine synovial cellular phenotype to obtain cell type % and cell type abundance phenotype (CTAP)1. The 6 CTAPs identified named by the dominant cell type were: (1) endothelial, fibroblast, and myeloid cells (EFM), (2) fibroblasts (F), (3) myeloid, (4) T- and B-cells (TB), (5) T cells and fibroblasts (TF), (6) T and myeloid cells (TM. We performed correlations between key RA clinical data (Figure 1) and cell type % using Spearman's correlation for continuous variables and point bi-seral for binary variables. Chi square or ANOVA were performed to test differences in means or proportions, respectively across CTAP categories. In this discovery cohort, we focused on associations p< 0.01, and reported p< 0.05.
Results: We studied n=105 subjects, mean age 56 years, 74% female, 70% White, 15% Black, 31% Hispanic; 85% were seropositive, mean RA duration 6.6 years, DAS28-CRP3 4.8, and hsCRP 18.1mg/L. Anti-CCP and RF positivity correlated with low % myeloid cells, while RF+ was correlated with high % T-cells (Figure 1). Higher DAS28-CRP was correlated with higher % T cells in the synovium. Across CTAPs, the TF cluster had the highest HAQ and patient global scores (Table 2). When examining variation across treatments, subjects on MTX or other nbDMARDs alone had higher %B-cells than other groups. For subjects on bDMARDs, the majority belonged to the EFM CTAP (57%), while none were categorized as TF. No significant difference was observed across CTAPs for age, sex, RA disease duration, DAS28-CRP, and hsCRP.
Conclusion: In this comprehensive screen of clinical factors measured at the time of synovial biopsy, we observed differential associations between cell phenotypes with bDMARD vs nbDMARD use, suggesting that RA therapies may impact cell composition in the synovium. Future directions include longitudinal studies of treatment response to determine whether cell phenotypes can improve our ability to select the optimal RA treatment for each patient.
1Zhang, Jonsson, et al., bioRxiv 2022.02.25.481990
 Figure 1. Correlations between cell type % with clinical factors; p-value < 0.01 in green, p≥0.01 and < 0.05 in blue.
Figure 1. Correlations between cell type % with clinical factors; p-value < 0.01 in green, p≥0.01 and < 0.05 in blue..jpg)
Disclosures: D. Weisenfeld, None; F. Zhang, None; L. Donlin, None; A. Jonsson, Amgen; W. Apruzzese, None; D. Campbell, None; E. Gravallese, New England Journal of Medicine, Textbook Rheumatology, AMN healthcare, CVS, American Well Corporation, Cerner Corp; L. Moreland, None; S. Goodman, Novartis, UCB; M. Brenner, GSK, 4FO Ventures, Mestag Therapeutics; S. Raychaudhuri, Mestag, Inc, Rheos Medicines, Janssen, Pfizer, Biogen; A. Filer, None; J. Anolik, None; V. Bykerk, Amgen, Bristol-Myers Squibb(BMS), Genzyme, Brainstorm, Gilead, Regeneron, UCB, Pfizer, Sanofi, Aventis; K. Liao, None.

