Back
Plenary Session
Juvenile idiopathic arthritis (JIA) and pediatric joint disorders
Session: Plenary III
1679: The Childhood Arthritis and Rheumatology Research Alliance Start Time Optimization of Biologic Therapy in Polyarticular JIA (STOP-JIA) Study: Three-Year Outcomes
Monday, November 14, 2022
12:30 PM – 12:40 PM Eastern Time
Location: Exhibit Hall A
.png)
Yukiko Kimura, MD
Hackensack Meridian School of Medicine
New York, NY, United States
Presenting Author(s)
Yukiko Kimura1, Sarah Ringold2, George Tomlinson3, Laura Schanberg4, Anne Dennos5, MaryEllen Riordan6, Vincent Del Gaizo7, Katherine Murphy8, Pamela Weiss9, Brian Feldman10, Mei Sing Ong11 and Marc Natter12, 1Hackensack Meridian Health, New York, NY, 2Janssen, Seattle, WA, 3University of Toronto, Toronto, ON, Canada, 4Duke University Medical Center, Durham, NC, 5Duke University, Durham, NC, 6Hackensack Meridian Health, Hackensack, NJ, 7CARRA, Inc, Washington, DC, 8CARRA, Inc, New Orleans, LA, 9Children's Hospital of Philadelphia, Glen Mills, PA, 10Division of Rheumatology, The Hospital for Sick Children; Child Health Evaluative Services, SickKids Research Institute; Department of Paediatrics, University of Toronto, Toronto, ON, Canada, 11Harvard Pilgrim Institute, Boston, MA, 12Boston Children's Hospital, Boston, MA
Background/Purpose: The CARRA STOP-JIA study compared the effectiveness of the CARRA Consensus Treatment Plans (CTPs) in achieving clinically inactive disease (CID) in untreated polyarticular JIA (pJIA) using a prospective, observational study design. The primary results at 12 months, and follow-up at 2 years, were reported previously. Longer-term follow-up of this cohort is ongoing through the CARRA Registry. Here we report on the impact of initial CTP choices on outcomes at 3 years.
Methods: STOP-JIA compared 3 CARRA CTPs in untreated pJIA patients: 1) Step Up (SU) – starting conventional synthetic DMARD (csDMARD), adding biologic (b)DMARD if needed after ≥ 3 months; 2) Early Combination (EC) - csDMARD and bDMARD started together; and 3) Biologic First (BF) – starting bDMARD monotherapy. There was no randomization. Data were collected using the CARRA Registry every 3 months for the first 12 months and every 6 months (+/- 3 months) thereafter. Patients with 36 (+/- 6) months of follow-up were included in this analysis. The proportion of children achieving Clinically Inactive Disease (CID) off glucocorticoids (GC), the clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS10) inactive disease, and Clinical Remission (CID for ≥6 months) were evaluated at 3 years. In addition, the percentage of time participants in each CTP group spent in CID and cJADAS10 inactive disease (ID) (score ≤2.5) were calculated by assuming disease status was constant between visits and taking the participant's total time in the inactive state divided by the total follow-up time. Propensity score (PS) weighting was used to balance baseline differences in potential confounders between CTPs, and multiple imputation (MI) was used to account for missing variables.
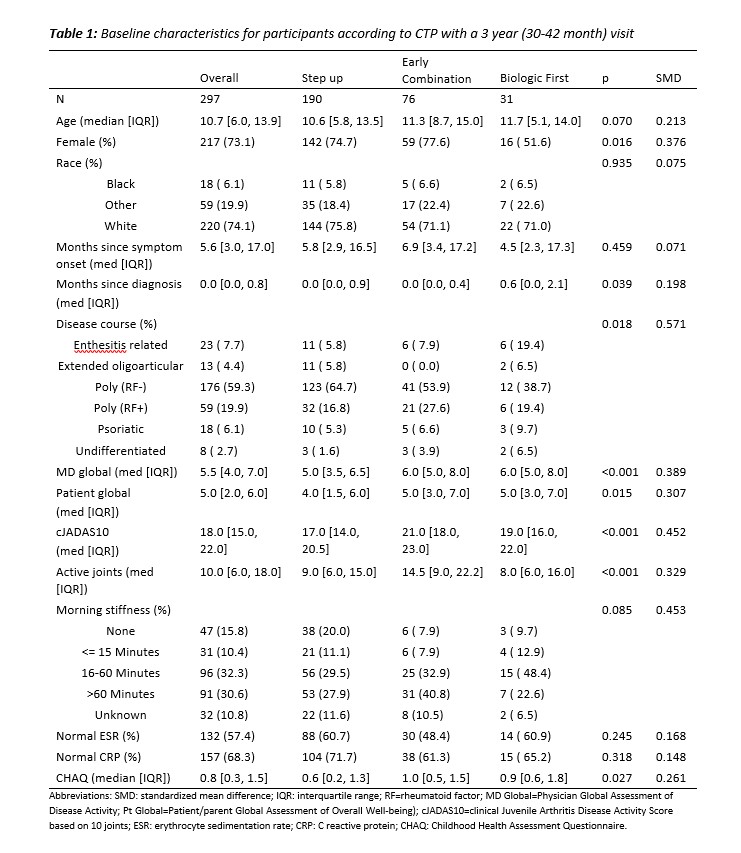
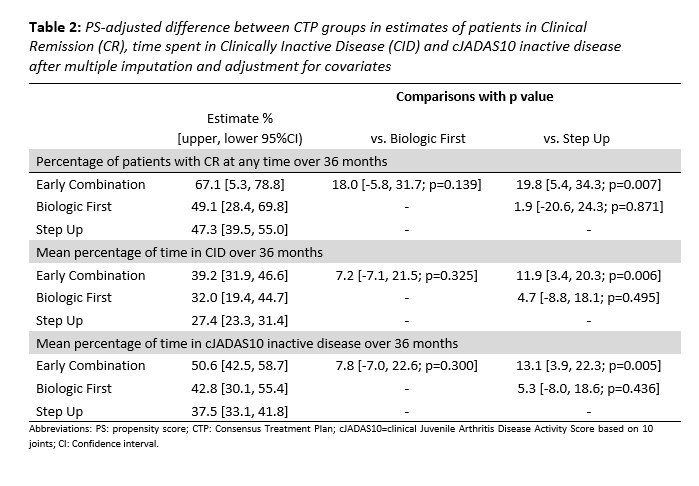
Results: 297 pJIA participants had a 3-year visit (190 Step Up, 76 Early Combination, 31 Biologic First). There were some baseline differences between CTP groups for several variables as reported previously (Table 1). The characteristics did not differ significantly at baseline from the entire cohort (N=400) and those who missed a 3-year visit, except for older age at onset and a higher proportion of black race (in those missing the 3-year visit). At the 3-year timepoint, the percentage of patients in CID and cJADAS10 inactive disease (ID) (score ≤2.5) did not differ between the CTP groups. However, the proportion of patients who achieved Clinical Remission at any time in the study (EC: 67.1%, SU 47.3%; p=0.007), and the percentage of time patients in each group spent in both CID (EC: 39.2%, SU 27.3%; p=0.006) and cJADAS10 ID (EC: 50.6%, SU 37.5%; p=0.005) were each significantly higher in the EC group compared to the SU group (Table 2). Figure 1 shows the proportion of patients in each cJADAS10 category by CTP group over the 3-year period.
Conclusion: Although there were no significant differences between pJIA patients in the 3 CTP groups in achieving CID or cJADAS10 ID at the 3 year timepoint, the Early Combination CTP group had a significantly higher proportion of patients who were able to achieve Clinical Remission, and significantly longer times spent in CID and cJADAS10 ID during the 3 year study period compared to the Step Up CTP group.
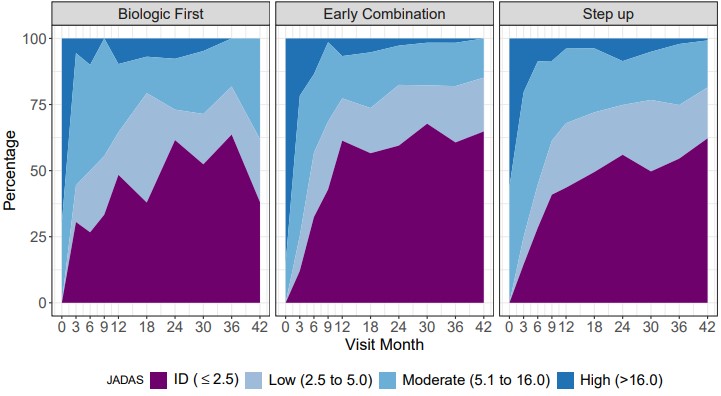 cJADAS10 disease activity categories for each Consensus Treatment Plan group over the 3-year time period.
cJADAS10 disease activity categories for each Consensus Treatment Plan group over the 3-year time period.
Disclosures: Y. Kimura, Genentech; S. Ringold, Janssen; G. Tomlinson, None; L. Schanberg, UCB, Sanofi; A. Dennos, None; M. Riordan, None; V. Del Gaizo, None; K. Murphy, None; P. Weiss, None; B. Feldman, Pfizer, AB2-Bio, Janssen; M. Ong, None; M. Natter, None.
Background/Purpose: The CARRA STOP-JIA study compared the effectiveness of the CARRA Consensus Treatment Plans (CTPs) in achieving clinically inactive disease (CID) in untreated polyarticular JIA (pJIA) using a prospective, observational study design. The primary results at 12 months, and follow-up at 2 years, were reported previously. Longer-term follow-up of this cohort is ongoing through the CARRA Registry. Here we report on the impact of initial CTP choices on outcomes at 3 years.
Methods: STOP-JIA compared 3 CARRA CTPs in untreated pJIA patients: 1) Step Up (SU) – starting conventional synthetic DMARD (csDMARD), adding biologic (b)DMARD if needed after ≥ 3 months; 2) Early Combination (EC) - csDMARD and bDMARD started together; and 3) Biologic First (BF) – starting bDMARD monotherapy. There was no randomization. Data were collected using the CARRA Registry every 3 months for the first 12 months and every 6 months (+/- 3 months) thereafter. Patients with 36 (+/- 6) months of follow-up were included in this analysis. The proportion of children achieving Clinically Inactive Disease (CID) off glucocorticoids (GC), the clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS10) inactive disease, and Clinical Remission (CID for ≥6 months) were evaluated at 3 years. In addition, the percentage of time participants in each CTP group spent in CID and cJADAS10 inactive disease (ID) (score ≤2.5) were calculated by assuming disease status was constant between visits and taking the participant's total time in the inactive state divided by the total follow-up time. Propensity score (PS) weighting was used to balance baseline differences in potential confounders between CTPs, and multiple imputation (MI) was used to account for missing variables.
Results: 297 pJIA participants had a 3-year visit (190 Step Up, 76 Early Combination, 31 Biologic First). There were some baseline differences between CTP groups for several variables as reported previously (Table 1). The characteristics did not differ significantly at baseline from the entire cohort (N=400) and those who missed a 3-year visit, except for older age at onset and a higher proportion of black race (in those missing the 3-year visit). At the 3-year timepoint, the percentage of patients in CID and cJADAS10 inactive disease (ID) (score ≤2.5) did not differ between the CTP groups. However, the proportion of patients who achieved Clinical Remission at any time in the study (EC: 67.1%, SU 47.3%; p=0.007), and the percentage of time patients in each group spent in both CID (EC: 39.2%, SU 27.3%; p=0.006) and cJADAS10 ID (EC: 50.6%, SU 37.5%; p=0.005) were each significantly higher in the EC group compared to the SU group (Table 2). Figure 1 shows the proportion of patients in each cJADAS10 category by CTP group over the 3-year period.
Conclusion: Although there were no significant differences between pJIA patients in the 3 CTP groups in achieving CID or cJADAS10 ID at the 3 year timepoint, the Early Combination CTP group had a significantly higher proportion of patients who were able to achieve Clinical Remission, and significantly longer times spent in CID and cJADAS10 ID during the 3 year study period compared to the Step Up CTP group.


 cJADAS10 disease activity categories for each Consensus Treatment Plan group over the 3-year time period.
cJADAS10 disease activity categories for each Consensus Treatment Plan group over the 3-year time period.Disclosures: Y. Kimura, Genentech; S. Ringold, Janssen; G. Tomlinson, None; L. Schanberg, UCB, Sanofi; A. Dennos, None; M. Riordan, None; V. Del Gaizo, None; K. Murphy, None; P. Weiss, None; B. Feldman, Pfizer, AB2-Bio, Janssen; M. Ong, None; M. Natter, None.

