Back
Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0343–0371) SLE – Treatment Poster I
0344: Therapeutic Thresholds of Hydroxychloroquine Blood Levels: Physiologic and Social Determinants of Low Hydroxychloroquine Blood Levels
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- SG
Shivani Garg, MD, MS
University of Madison, School of Medicine and Public Health
Madison, WI, United States
Abstract Poster Presenter(s)
Shivani Garg1, Betty Chewning2, Brad Astor3 and Christie Bartels4, 1University of Madison, School of Medicine and Public Health, Madison, WI, 2University of Wisconsin, School of Pharmacy, Madison, WI, 3University of Wisconsin, School of Medicine and Public Health, Madison, WI, 4University of Wisconsin School of Medicine and Public Health, Madison, WI
Background/Purpose: Lupus, the leading cause of chronic kidney disease (CKD) in young women, is treated with Hydroxychloroquine (HCQ) which is primarily excreted by kidneys. Yet how physiologic renal function impacts HCQ levels is unclear, and highly relevant for safety and efficacy. Lupus damage shows disparities suggesting that adverse social determinants may influence outcomes, possibly via low HCQ levels. Finally, due to misperceived risks vs. benefits, up to 83% of patients may stop HCQ highlighting the need to clarify HCQ thresholds for adherence, efficacy, and safety. We aimed to: i) examine how renal function, dose, and other factors predict change in HCQ levels, ii) clarify thresholds of HCQ levels to predict adherence, efficacy, and identify patients at-risk for toxicity.
Methods: We measured HCQ levels with liquid chromatography mass spectrometry on whole blood from consented adults with lupus on HCQ therapy for ≥1 month. We abstracted HCQ dose by weight, sociodemographics, SLE disease activity index (SLEDAI ≥6 defined lupus flare), glomerular filtration rate (GFR), CKD stage, and data on adverse social determinants: Medicaid, disadvantaged neighborhoods by area deprivation index, and reported social barriers. Patients reported adherence on a 0-100% MASRI visual analogue scale (≥80% defined adherence).
Multivariable mixed regression models assessed how renal function, adverse social determinants, and other factors predict change in HCQ levels. Based on prior studies, a clinically significant change in HCQ levels was ≥8% change in mean levels (≥64.5 ng/mL based on maximal diurnal variation). We tested four levels, ≥500, ≥750, ≥1000, and ≥1500 ng/mL, to examine thresholds of HCQ levels that indicate adherence, efficacy, and identify higher toxicity risk.
Results: The mean HCQ level across 166 samples was 806±477 ng/ml. We found that CKD stage ≥2 was associated with a clinically significant increase in HCQ levels of 149 ng/mL vs. stage 1 (Table 1). Similar findings were noted when GFR was used instead of CKD stage (Table 2).
We noted reported adherence ≥80% was associated with a 646 ng/mL increase in HCQ levels vs. < 80% adherence. Finally, 400 mg/day prescribed dose increased HCQ levels by 448 ng/mL vs. 200 mg/day after even controlling for doses >5mg/kg/day. We found that increase in body weight by 15 kg was associated with a decrease in HCQ levels by 82 ng/mL (Table 1). Adverse social determinants did not predict change in HCQ levels.
HCQ levels ≥500 ng/mL predicted 21-fold higher odds of patient-reported adherence (Table 3A). Both ≥750 ng/ml & ≥1000 ng/mL thresholds predicted ~75% lower odds of flare (Table 3B-C). We found 4-fold higher odds of HCQ levels ≥1500 ng/mL in patients with CKD stage ≥2 (Table 3D).
Conclusion: Our findings inform practice change by clarifying clinical thresholds and predictors of changes in HCQ levels. Our study is the first to report that CKD stage ≥2 predicted 4-fold higher odds of supratherapeutic levels (≥1500 ng/mL), highlighting a need for monitoring levels in CKD. Adverse social determinants did not predict lower levels. Our study validates HCQ levels ≥500 ng/mL as a good threshold to detect adherence, and HCQ levels of 750-1000 ng/mL to prevent 75% flares.
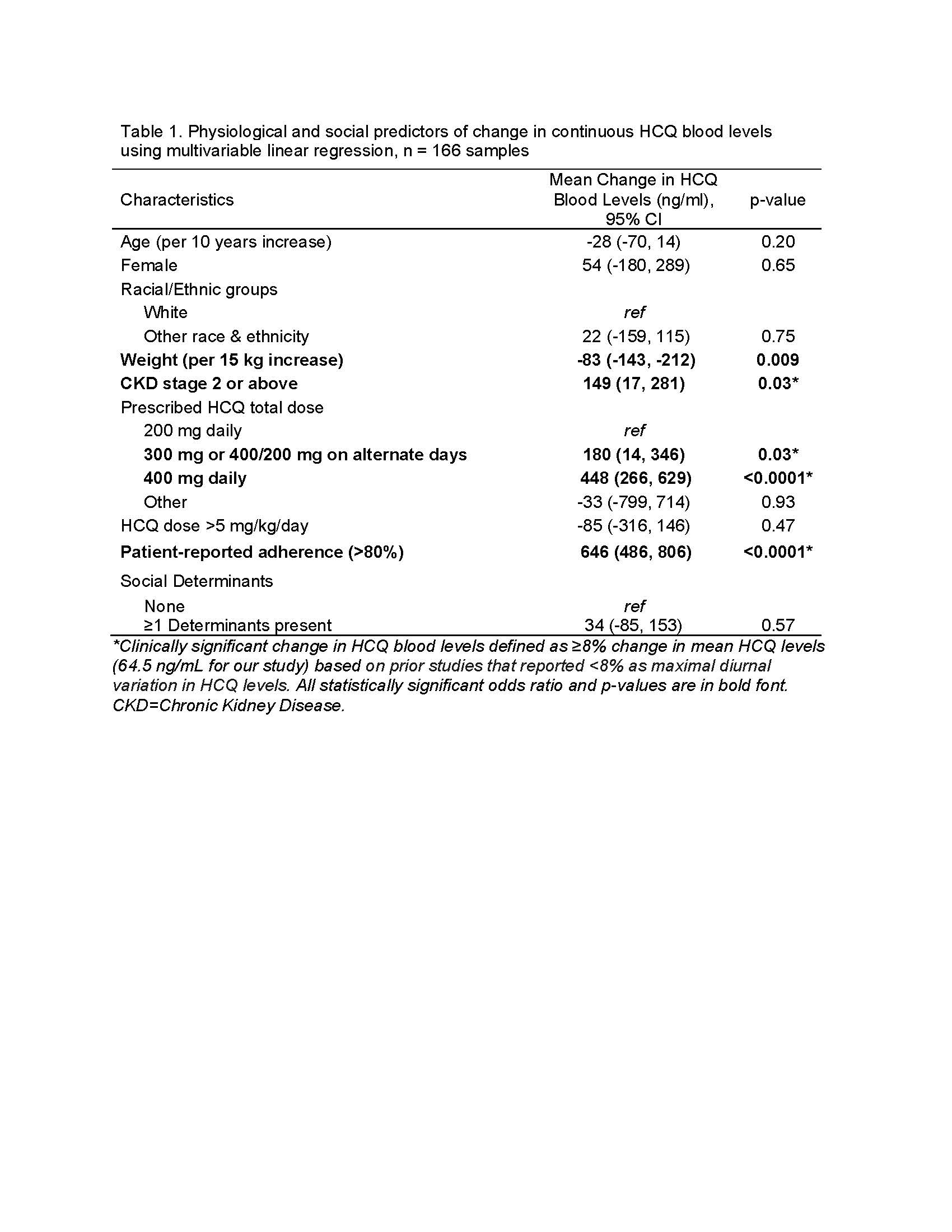 Table 1. Physiological and social predictors of change in continuous HCQ blood levels using multivariable linear regression, n = 166 samples
Table 1. Physiological and social predictors of change in continuous HCQ blood levels using multivariable linear regression, n = 166 samples
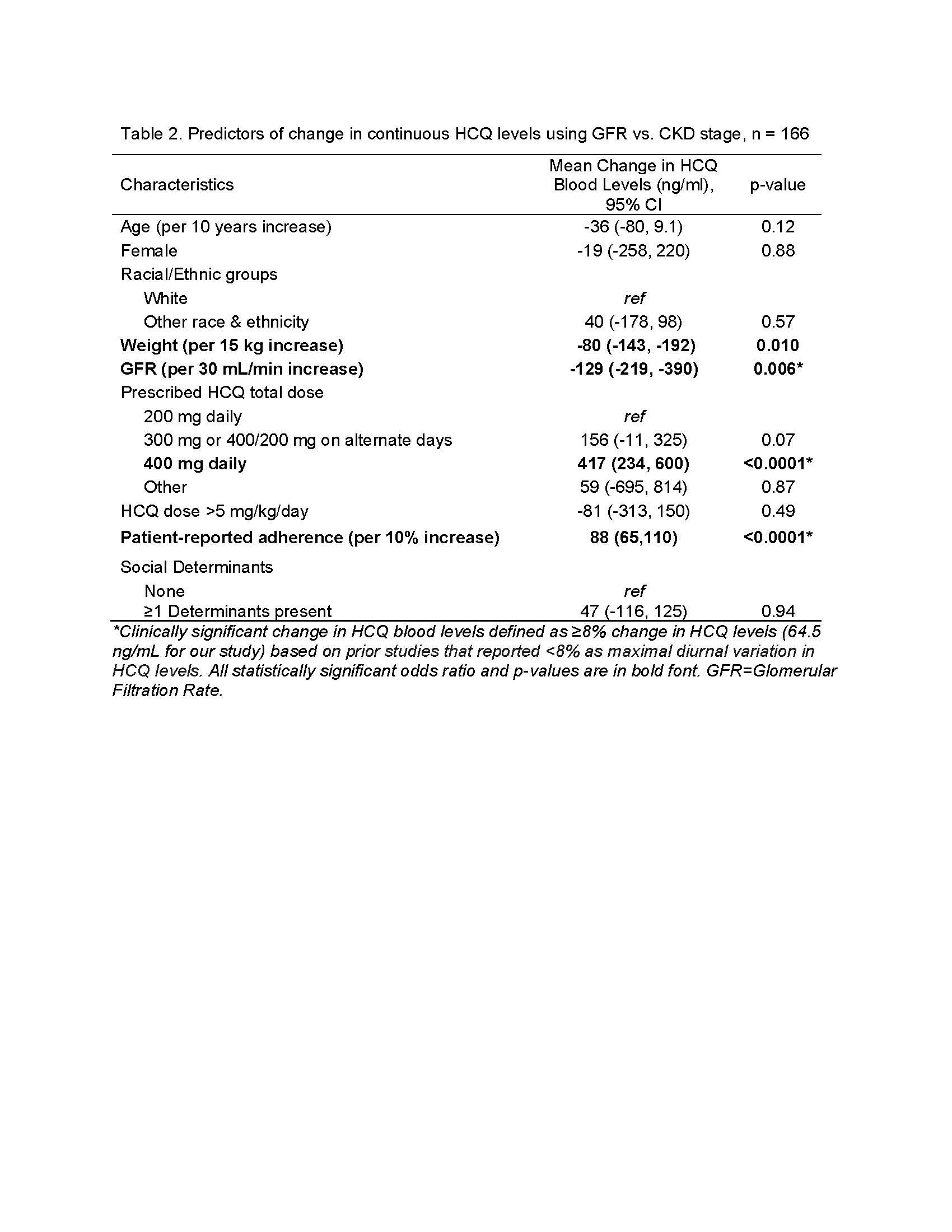 Table 2. Predictors of change in continuous HCQ levels using GFR vs. CKD stage, n = 166
Table 2. Predictors of change in continuous HCQ levels using GFR vs. CKD stage, n = 166
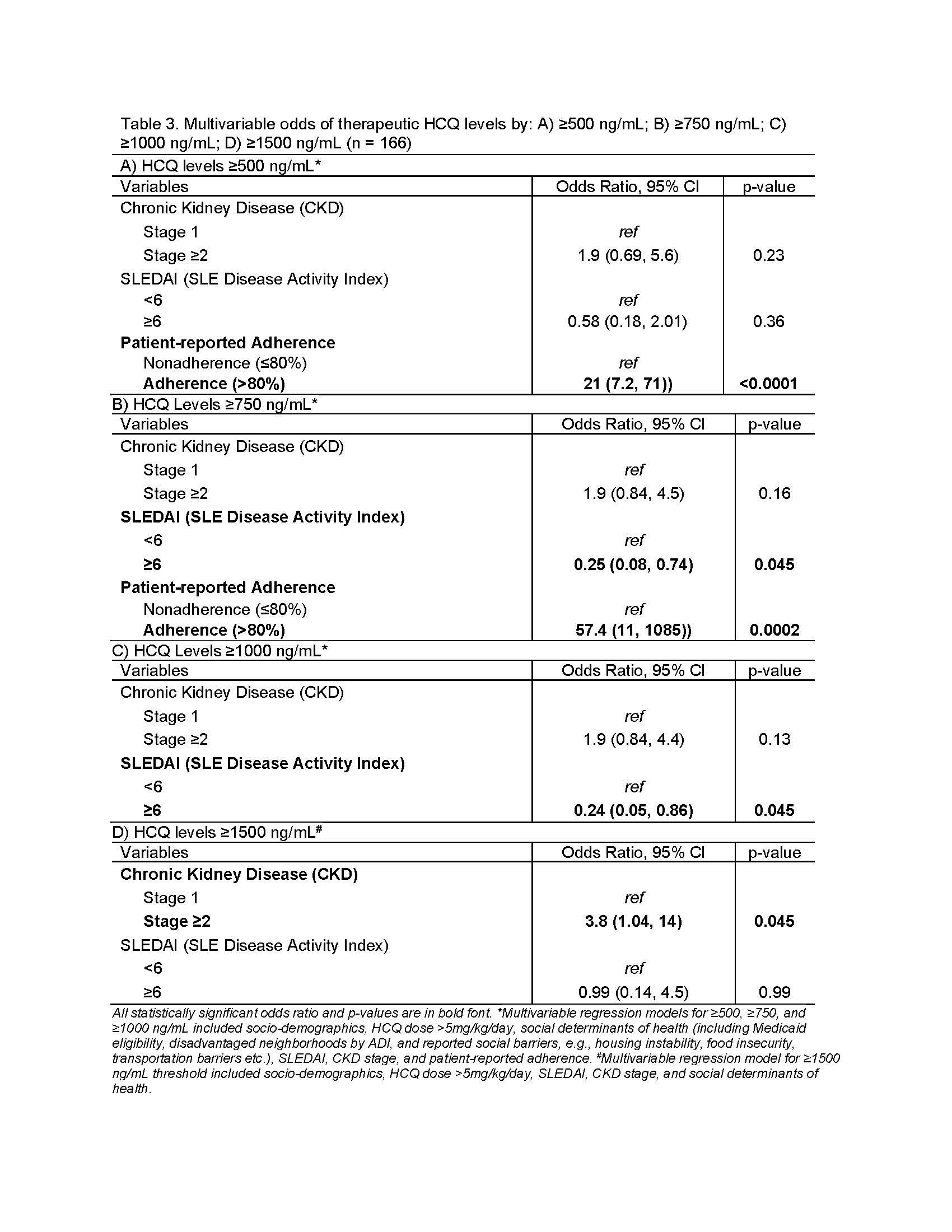 Table 3. Multivariable odds of therapeutic HCQ levels by: A) ≥500 ng/mL; B) ≥750 ng/mL; C) ≥1000 ng/mL; D) ≥1500 ng/mL (n = 166)
Table 3. Multivariable odds of therapeutic HCQ levels by: A) ≥500 ng/mL; B) ≥750 ng/mL; C) ≥1000 ng/mL; D) ≥1500 ng/mL (n = 166)
Disclosures: S. Garg, None; B. Chewning, None; B. Astor, None; C. Bartels, Pfizer.
Background/Purpose: Lupus, the leading cause of chronic kidney disease (CKD) in young women, is treated with Hydroxychloroquine (HCQ) which is primarily excreted by kidneys. Yet how physiologic renal function impacts HCQ levels is unclear, and highly relevant for safety and efficacy. Lupus damage shows disparities suggesting that adverse social determinants may influence outcomes, possibly via low HCQ levels. Finally, due to misperceived risks vs. benefits, up to 83% of patients may stop HCQ highlighting the need to clarify HCQ thresholds for adherence, efficacy, and safety. We aimed to: i) examine how renal function, dose, and other factors predict change in HCQ levels, ii) clarify thresholds of HCQ levels to predict adherence, efficacy, and identify patients at-risk for toxicity.
Methods: We measured HCQ levels with liquid chromatography mass spectrometry on whole blood from consented adults with lupus on HCQ therapy for ≥1 month. We abstracted HCQ dose by weight, sociodemographics, SLE disease activity index (SLEDAI ≥6 defined lupus flare), glomerular filtration rate (GFR), CKD stage, and data on adverse social determinants: Medicaid, disadvantaged neighborhoods by area deprivation index, and reported social barriers. Patients reported adherence on a 0-100% MASRI visual analogue scale (≥80% defined adherence).
Multivariable mixed regression models assessed how renal function, adverse social determinants, and other factors predict change in HCQ levels. Based on prior studies, a clinically significant change in HCQ levels was ≥8% change in mean levels (≥64.5 ng/mL based on maximal diurnal variation). We tested four levels, ≥500, ≥750, ≥1000, and ≥1500 ng/mL, to examine thresholds of HCQ levels that indicate adherence, efficacy, and identify higher toxicity risk.
Results: The mean HCQ level across 166 samples was 806±477 ng/ml. We found that CKD stage ≥2 was associated with a clinically significant increase in HCQ levels of 149 ng/mL vs. stage 1 (Table 1). Similar findings were noted when GFR was used instead of CKD stage (Table 2).
We noted reported adherence ≥80% was associated with a 646 ng/mL increase in HCQ levels vs. < 80% adherence. Finally, 400 mg/day prescribed dose increased HCQ levels by 448 ng/mL vs. 200 mg/day after even controlling for doses >5mg/kg/day. We found that increase in body weight by 15 kg was associated with a decrease in HCQ levels by 82 ng/mL (Table 1). Adverse social determinants did not predict change in HCQ levels.
HCQ levels ≥500 ng/mL predicted 21-fold higher odds of patient-reported adherence (Table 3A). Both ≥750 ng/ml & ≥1000 ng/mL thresholds predicted ~75% lower odds of flare (Table 3B-C). We found 4-fold higher odds of HCQ levels ≥1500 ng/mL in patients with CKD stage ≥2 (Table 3D).
Conclusion: Our findings inform practice change by clarifying clinical thresholds and predictors of changes in HCQ levels. Our study is the first to report that CKD stage ≥2 predicted 4-fold higher odds of supratherapeutic levels (≥1500 ng/mL), highlighting a need for monitoring levels in CKD. Adverse social determinants did not predict lower levels. Our study validates HCQ levels ≥500 ng/mL as a good threshold to detect adherence, and HCQ levels of 750-1000 ng/mL to prevent 75% flares.
 Table 1. Physiological and social predictors of change in continuous HCQ blood levels using multivariable linear regression, n = 166 samples
Table 1. Physiological and social predictors of change in continuous HCQ blood levels using multivariable linear regression, n = 166 samples Table 2. Predictors of change in continuous HCQ levels using GFR vs. CKD stage, n = 166
Table 2. Predictors of change in continuous HCQ levels using GFR vs. CKD stage, n = 166  Table 3. Multivariable odds of therapeutic HCQ levels by: A) ≥500 ng/mL; B) ≥750 ng/mL; C) ≥1000 ng/mL; D) ≥1500 ng/mL (n = 166)
Table 3. Multivariable odds of therapeutic HCQ levels by: A) ≥500 ng/mL; B) ≥750 ng/mL; C) ≥1000 ng/mL; D) ≥1500 ng/mL (n = 166) Disclosures: S. Garg, None; B. Chewning, None; B. Astor, None; C. Bartels, Pfizer.

