Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0272–0316) RA – Treatment Poster I
0275: Exploratory Analysis of Filgotinib Safety Data in Patients with Moderately to Severely Active RA and an Increased Risk of Cardiovascular Events: Data from Phase 2 and 3 Clinical Trials
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
.png)
Maya Buch, MD, PhD
University of Manchester
Manchester, United Kingdom
Abstract Poster Presenter(s)
Maya Buch1, Gerd Burmester2, Xavier Mariette3, Christina Charles-Schoeman4, Vijay Rajendran5, Pieter-Jan Stiers6, Agustin Cerani6, Paul Van Hoek6, Katrien Van Beneden6, Yoshiya Tanaka7, Hendrik Schulze-Koops8, Rene Westhovens9 and Ennio Giulio Favalli10, 1University of Manchester and NIHR Manchester Biomedical Research Centre, Manchester, United Kingdom, 2Charité University Medicine Berlin, Berlin, Germany, 3Paris-Saclay University, Rueil Malmaison, Ile-de-France, France, 4Division of Rheumatology, University of California, Los Angeles, Santa Monica, CA, 5Galapagos NV, Gent, Belgium, 6Galapagos NV, Mechelen, Belgium, 7University of Occupational and Environmental Health, Kitakyusyu Fukuoka, Japan, 8Ludwig-Maximilians-University Munich, Munich, Germany, 9University Hospitals Leuven, Leuven, Belgium, 10University of Milan, ASST Gaetano Pini-CTO Institute, Milano, Italy
Background/Purpose: The safety profile of filgotinib (FIL), a second-generation oral Janus kinase (JAK) 1 preferential inhibitor approved in Europe, Japan, and the UK for treatment of RA,1,2 has been reported.3 In patients (pts) with active RA aged ≥50 y with ≥1 cardiovascular (CV) risk factor, treated with the pan-JAK inhibitor tofacitinib, data from an interventional post-marketing study (NCT02092467)4 suggested a higher risk of major adverse cardiovascular events (MACE) and malignancies compared with TNF inhibitors. No data are available from a similar prospective study with FIL. This post hoc exploratory analysis aimed to describe the incidence of MACE and malignancies in a subgroup of pts with RA from the FINCH and DARWIN clinical trials, receiving FIL 200 mg (FIL200) and FIL 100 mg (FIL100).
Methods: Exploratory analysis of adverse events of special interest are reported using integrated FIL RA data from phase 2 (NCT01668641, NCT01894516), phase 3 (NCT02889796, NCT02873936, NCT02886728), and the long-term extension (LTE) studies DARWIN 3 phase 2 (NCT02065700) and FINCH 4 phase 3 (NCT03025308), in a pt population at higher risk of CV events similar to ORAL-SURVEILLANCE,1 namely, aged ≥50 y with ≥1 CV risk factor (history of dyslipidemia, diabetes or CV disease; hypertension, ischemic vascular conditions, peripheral vascular disease, extra-articular manifestations of RA; or current smokers). All pts met ACR criteria for functional class I–III. Censored exposure-adjusted incidence rates (EAIRs)/100 pt-years of exposure for MACE, venous thromboembolism (VTE), malignancies excluding nonmelanoma skin cancer (NMSC), NMSC, (serious) infections, and deaths were determined. Data were as of Jan 11, 2022 (DARWIN 3) and Jan 31, 2022 (FINCH 4). Analyses were performed on an ad hoc interim analysis data set without additional cleaning.
Results: The higher-risk population included 1484 pts: mean age 62.1 y (536 [36.1%] aged ≥65 y), 291 (22.5%) current smokers, and 904 (60.9%) received background MTX; baseline (BL) MTX use was higher in the FIL100 vs FIL200 group (66.6% vs 54.6%); other BL demographics were balanced. Numerically higher EAIRs for malignancies (excluding NMSC), NMSC, serious infections, and deaths were observed for pts receiving FIL200 vs FIL100; 95% confidence intervals overlapped (Table). For MACE and VTE, incidence is considered similar for both doses (Table). The risk of experiencing MACE, malignancies excluding NMSC, and NMSC over time with FIL100 or FIL200 is shown in the Figure.
Conclusion: In this RA population at higher risk of CV events, the incidence of MACE and VTE was similar for both doses. A numerically higher incidence of malignancies (excluding NMSC), NMSC, serious infections, and deaths was observed in the high- vs low-dose group. Limitations included population selection bias, low event numbers, and the post hoc nature of the analysis. The ongoing LTE and real-world studies in RA will continue to investigate the safety of FIL in these populations.
References
1. Jyseleca SmPC. Galapagos NV; May 2022
2. Jyseleca Japanese PI. Gilead Sciences K.K.; Sep 2020
3. Winthrop KL, et al. Ann Rheum Dis 2022;81:184–92
4. Ytterberg SR, et al. N Engl J Med 2022;386:316–26
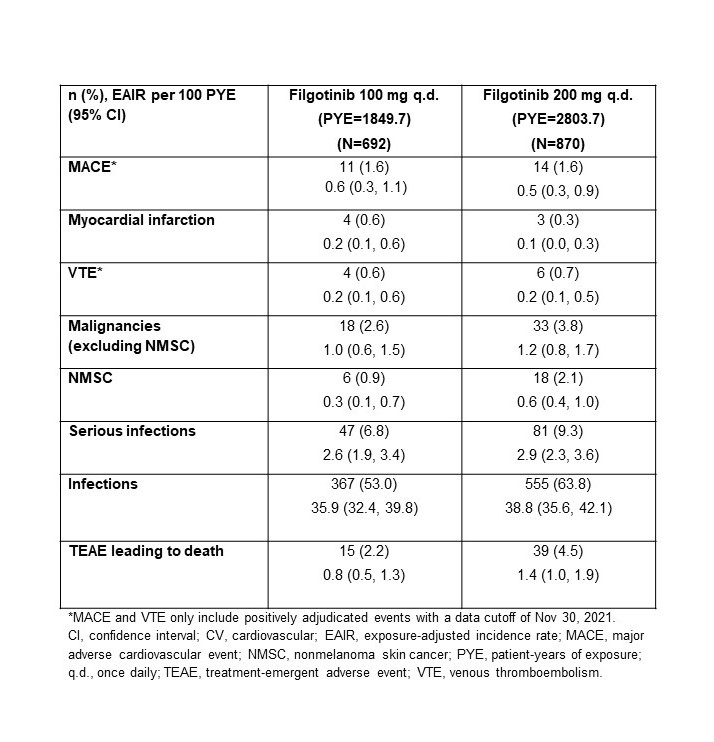 Table. Frequency and EAIR of treatment-emergent adverse events of special interest in a population of patients with RA at higher risk of CV events (aged ≥50 y, ≥1 CV risk)
Table. Frequency and EAIR of treatment-emergent adverse events of special interest in a population of patients with RA at higher risk of CV events (aged ≥50 y, ≥1 CV risk)
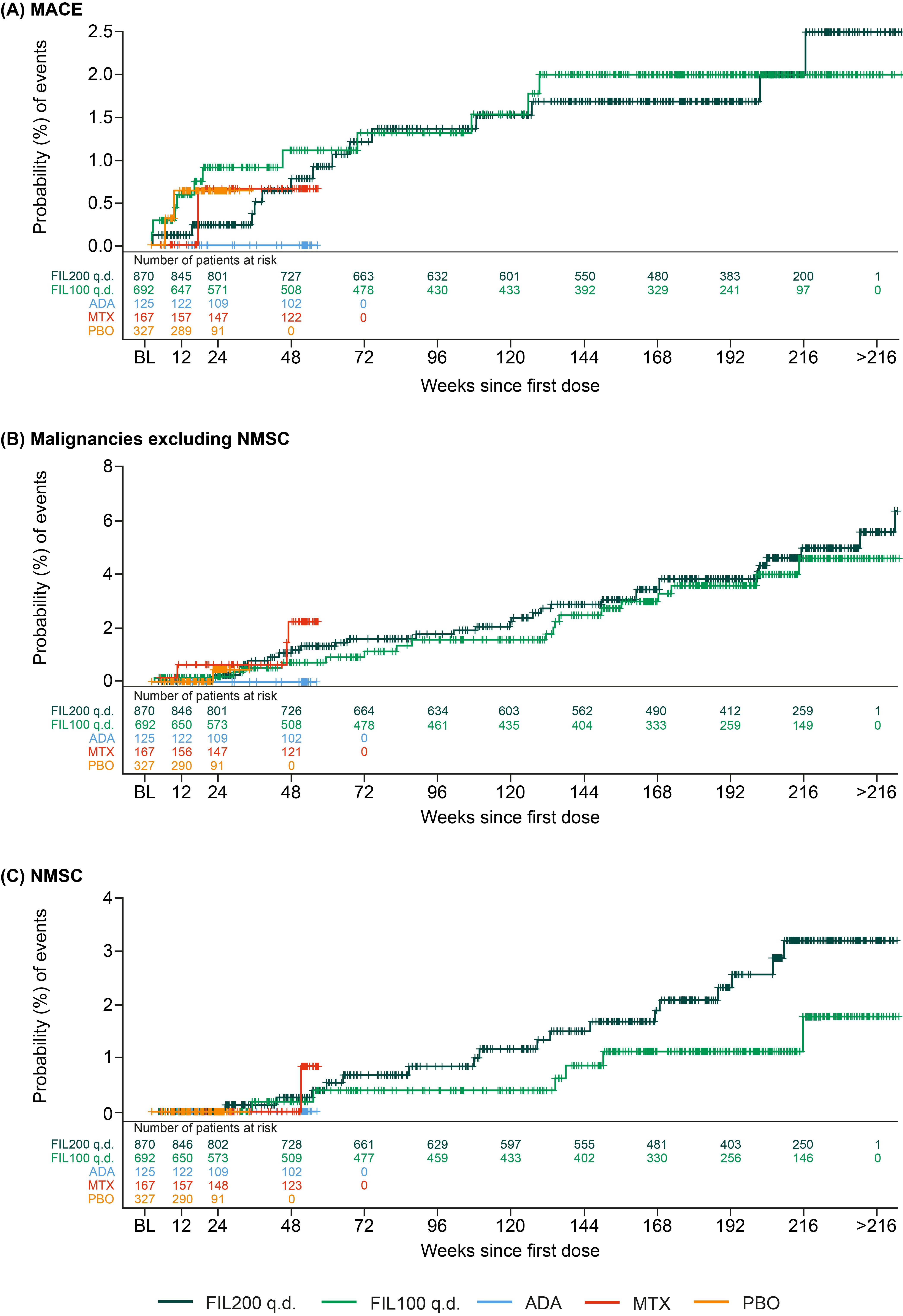 Figure. Time to first treatment-emergent event of (A) MACE, (B) malignancies excluding NMSC, and (C) NMSC in patients with RA who were aged ≥50 y with ≥1 CV risk factor. The time to event was calculated as (onset date of first event – first dose date +1); CV risk factors: Information on smoking habits and CV family history were not available in DARWIN 1–3. The population was as-treated, including patients who received ≥1 dose of any study drug. A patient may contribute to ≥1 treatment group summary if the patient received ≥1 treatment of interest. Number at risk = number of patients at risk at that the given timepoint. ADA, adalimumab; BL, baseline; CV, cardiovascular; FIL200/100, filgotinib 200/100 mg; MACE, major adverse cardiovascular event; MTX, methotrexate; NMSC, nonmelanoma skin cancer; PBO, placebo; q.d., once daily. Numbers of patients assessed at each time point are shown below each graph.
Figure. Time to first treatment-emergent event of (A) MACE, (B) malignancies excluding NMSC, and (C) NMSC in patients with RA who were aged ≥50 y with ≥1 CV risk factor. The time to event was calculated as (onset date of first event – first dose date +1); CV risk factors: Information on smoking habits and CV family history were not available in DARWIN 1–3. The population was as-treated, including patients who received ≥1 dose of any study drug. A patient may contribute to ≥1 treatment group summary if the patient received ≥1 treatment of interest. Number at risk = number of patients at risk at that the given timepoint. ADA, adalimumab; BL, baseline; CV, cardiovascular; FIL200/100, filgotinib 200/100 mg; MACE, major adverse cardiovascular event; MTX, methotrexate; NMSC, nonmelanoma skin cancer; PBO, placebo; q.d., once daily. Numbers of patients assessed at each time point are shown below each graph.
Disclosures: M. Buch, AbbVie, Galapagos, Gilead, Pfizer, Eli Lilly, Merck-Serono, Roche, UCB; G. Burmester, AbbVie, Galapagos, Lilly, MSD, Pfizer, Roche, UCB, Janssen, Gilead Sciences, Inc.; X. Mariette, AstraZeneca, Bristol Myers Squibb, Galapagos, GSK, Novartis, Pfizer; C. Charles-Schoeman, AbbVie, Bristol Myers Squibb (BMS), Pfizer, Regeneron-Sanofi, Gilead; V. Rajendran, Galapagos; P. Stiers, Galapagos; A. Cerani, Galapagos; P. Van Hoek, Galapagos; K. Van Beneden, Galapagos; Y. Tanaka, Lilly, AbbVie, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Pfizer, Mitsubishi Tanabe, GlaxoSmithKline, Asahi Kasei, Takeda, Astellas, Janssen, Novartis, Sanofi, UCB, YL Biologics, MSD, Ono, Taisho Toyama, Celltrion, Gilead, Boehringer-Ingelheim, Corrona, Kowa, Amgen, AstraZeneca, AstraZeneca, Eli Lilly; H. Schulze-Koops, AbbVie, Galapagos, Lilly, Pfizer; R. Westhovens, Celltrion, Galapagos, Gilead; E. Favalli, AbbVie, BMS, Celltrion, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer, UCB.
Background/Purpose: The safety profile of filgotinib (FIL), a second-generation oral Janus kinase (JAK) 1 preferential inhibitor approved in Europe, Japan, and the UK for treatment of RA,1,2 has been reported.3 In patients (pts) with active RA aged ≥50 y with ≥1 cardiovascular (CV) risk factor, treated with the pan-JAK inhibitor tofacitinib, data from an interventional post-marketing study (NCT02092467)4 suggested a higher risk of major adverse cardiovascular events (MACE) and malignancies compared with TNF inhibitors. No data are available from a similar prospective study with FIL. This post hoc exploratory analysis aimed to describe the incidence of MACE and malignancies in a subgroup of pts with RA from the FINCH and DARWIN clinical trials, receiving FIL 200 mg (FIL200) and FIL 100 mg (FIL100).
Methods: Exploratory analysis of adverse events of special interest are reported using integrated FIL RA data from phase 2 (NCT01668641, NCT01894516), phase 3 (NCT02889796, NCT02873936, NCT02886728), and the long-term extension (LTE) studies DARWIN 3 phase 2 (NCT02065700) and FINCH 4 phase 3 (NCT03025308), in a pt population at higher risk of CV events similar to ORAL-SURVEILLANCE,1 namely, aged ≥50 y with ≥1 CV risk factor (history of dyslipidemia, diabetes or CV disease; hypertension, ischemic vascular conditions, peripheral vascular disease, extra-articular manifestations of RA; or current smokers). All pts met ACR criteria for functional class I–III. Censored exposure-adjusted incidence rates (EAIRs)/100 pt-years of exposure for MACE, venous thromboembolism (VTE), malignancies excluding nonmelanoma skin cancer (NMSC), NMSC, (serious) infections, and deaths were determined. Data were as of Jan 11, 2022 (DARWIN 3) and Jan 31, 2022 (FINCH 4). Analyses were performed on an ad hoc interim analysis data set without additional cleaning.
Results: The higher-risk population included 1484 pts: mean age 62.1 y (536 [36.1%] aged ≥65 y), 291 (22.5%) current smokers, and 904 (60.9%) received background MTX; baseline (BL) MTX use was higher in the FIL100 vs FIL200 group (66.6% vs 54.6%); other BL demographics were balanced. Numerically higher EAIRs for malignancies (excluding NMSC), NMSC, serious infections, and deaths were observed for pts receiving FIL200 vs FIL100; 95% confidence intervals overlapped (Table). For MACE and VTE, incidence is considered similar for both doses (Table). The risk of experiencing MACE, malignancies excluding NMSC, and NMSC over time with FIL100 or FIL200 is shown in the Figure.
Conclusion: In this RA population at higher risk of CV events, the incidence of MACE and VTE was similar for both doses. A numerically higher incidence of malignancies (excluding NMSC), NMSC, serious infections, and deaths was observed in the high- vs low-dose group. Limitations included population selection bias, low event numbers, and the post hoc nature of the analysis. The ongoing LTE and real-world studies in RA will continue to investigate the safety of FIL in these populations.
References
1. Jyseleca SmPC. Galapagos NV; May 2022
2. Jyseleca Japanese PI. Gilead Sciences K.K.; Sep 2020
3. Winthrop KL, et al. Ann Rheum Dis 2022;81:184–92
4. Ytterberg SR, et al. N Engl J Med 2022;386:316–26
 Table. Frequency and EAIR of treatment-emergent adverse events of special interest in a population of patients with RA at higher risk of CV events (aged ≥50 y, ≥1 CV risk)
Table. Frequency and EAIR of treatment-emergent adverse events of special interest in a population of patients with RA at higher risk of CV events (aged ≥50 y, ≥1 CV risk) Figure. Time to first treatment-emergent event of (A) MACE, (B) malignancies excluding NMSC, and (C) NMSC in patients with RA who were aged ≥50 y with ≥1 CV risk factor. The time to event was calculated as (onset date of first event – first dose date +1); CV risk factors: Information on smoking habits and CV family history were not available in DARWIN 1–3. The population was as-treated, including patients who received ≥1 dose of any study drug. A patient may contribute to ≥1 treatment group summary if the patient received ≥1 treatment of interest. Number at risk = number of patients at risk at that the given timepoint. ADA, adalimumab; BL, baseline; CV, cardiovascular; FIL200/100, filgotinib 200/100 mg; MACE, major adverse cardiovascular event; MTX, methotrexate; NMSC, nonmelanoma skin cancer; PBO, placebo; q.d., once daily. Numbers of patients assessed at each time point are shown below each graph.
Figure. Time to first treatment-emergent event of (A) MACE, (B) malignancies excluding NMSC, and (C) NMSC in patients with RA who were aged ≥50 y with ≥1 CV risk factor. The time to event was calculated as (onset date of first event – first dose date +1); CV risk factors: Information on smoking habits and CV family history were not available in DARWIN 1–3. The population was as-treated, including patients who received ≥1 dose of any study drug. A patient may contribute to ≥1 treatment group summary if the patient received ≥1 treatment of interest. Number at risk = number of patients at risk at that the given timepoint. ADA, adalimumab; BL, baseline; CV, cardiovascular; FIL200/100, filgotinib 200/100 mg; MACE, major adverse cardiovascular event; MTX, methotrexate; NMSC, nonmelanoma skin cancer; PBO, placebo; q.d., once daily. Numbers of patients assessed at each time point are shown below each graph.Disclosures: M. Buch, AbbVie, Galapagos, Gilead, Pfizer, Eli Lilly, Merck-Serono, Roche, UCB; G. Burmester, AbbVie, Galapagos, Lilly, MSD, Pfizer, Roche, UCB, Janssen, Gilead Sciences, Inc.; X. Mariette, AstraZeneca, Bristol Myers Squibb, Galapagos, GSK, Novartis, Pfizer; C. Charles-Schoeman, AbbVie, Bristol Myers Squibb (BMS), Pfizer, Regeneron-Sanofi, Gilead; V. Rajendran, Galapagos; P. Stiers, Galapagos; A. Cerani, Galapagos; P. Van Hoek, Galapagos; K. Van Beneden, Galapagos; Y. Tanaka, Lilly, AbbVie, Bristol Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Pfizer, Mitsubishi Tanabe, GlaxoSmithKline, Asahi Kasei, Takeda, Astellas, Janssen, Novartis, Sanofi, UCB, YL Biologics, MSD, Ono, Taisho Toyama, Celltrion, Gilead, Boehringer-Ingelheim, Corrona, Kowa, Amgen, AstraZeneca, AstraZeneca, Eli Lilly; H. Schulze-Koops, AbbVie, Galapagos, Lilly, Pfizer; R. Westhovens, Celltrion, Galapagos, Gilead; E. Favalli, AbbVie, BMS, Celltrion, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Pfizer, UCB.

