Back
Poster Session C
Pediatric autoimmune diseases: Kawasaki disease, juvenile dermatomyositis and juvenile localized scleroderma
Session: (1360–1386) Pediatric Rheumatology – Clinical Poster II: Connective Tissue Disease
1383: Differences in Clinical and Patient-reported Outcomes in Juvenile Dermatomyositis by Race and Ethnicity
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- RO
Rebecca Olveda, MD
UCSF
San Francisco, CA, United States
Abstract Poster Presenter(s)
Rebecca Olveda, Jessica Neely and Susan Kim, UCSF Benioff Children's Hospital, San Francisco, CA
Background/Purpose: Previous studies in juvenile dermatomyositis (JDM) have shown that patients from minoritized ethnicities and those with lower family income are more likely to have worse clinical outcomes. Patient-reported outcome measures (PROs) are valuable indicators of disease activity but are less well-studied in JDM. This study aimed to investigate differences in clinical outcomes and PROs based on race and ethnicity at baseline and at 12 months, in a cohort of patients enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry.
Methods: This was a retrospective cohort study using data from children diagnosed with JDM and enrolled into the CARRA Registry between February 2018 and November 2021, for whom data regarding race and ethnicity were available. The primary predictor was self-identified race and/or ethnicity. The primary outcome was the Patient/Parent Global Assessment Score (PGA) as a dichotomous variable of score < /= 2 (lower disease activity) or > 2 (higher disease activity). Secondary outcomes included proximal muscle weakness, elevated muscle enzymes, and PGA score at 12 months. Descriptive statistics were used to summarize each variable, and demographic and baseline disease activity measures were evaluated across racial and ethnic categories using Chi-squared and Kruskal-Wallis tests. Demographic variables with significant association with race and ethnicity were included in a multivariate model analyzing PGA score greater than 2 at baseline.
Results: 250 participants identified as Asian (5.6%), Black (11.2%), Hispanic (18.4%), or White (64.8%). Demographic characteristics are listed in Table 1. A higher proportion of Black and Hispanic participants were in the lowest income brackets (p = 0.04) (Table 1). At baseline, Asian, Black, and Hispanic participants were more likely to have proximal muscle weakness (p = 0.01) and elevated muscle enzymes compared to White participants (p = 0.04) (Table 2). Black participants had 3.1 times the odds of having a PGA > 2 at baseline compared to White participants (95% CI 1.2 to 7.8, p = 0.02). After adjusting for age at diagnosis, income, education, and insurance status, this association was no longer statistically significant. White participants tended to have lower PGA scores at baseline and at 12 months compared to Black and Asian participants. However, PGA score at 12 months and change in PGA score over 1 year were not significantly different between racial and ethnic groups (Figure 1).
Conclusion: In JDM participants enrolled in the CARRA Registry, Black and Hispanic participants tended to have lower income and education levels. While Black participants had a higher odds of having worse PGA at baseline, this association diminished after adjusting for age at diagnosis, income, education, and insurance status. Our study was limited by a smaller proportion of participants from minoritized racial and ethnic groups. More research is needed to determine how patient outcomes are influenced by social determinants of health, including income, education, and the effects of systemic racism, in order to inform future interventions to improve health disparities.
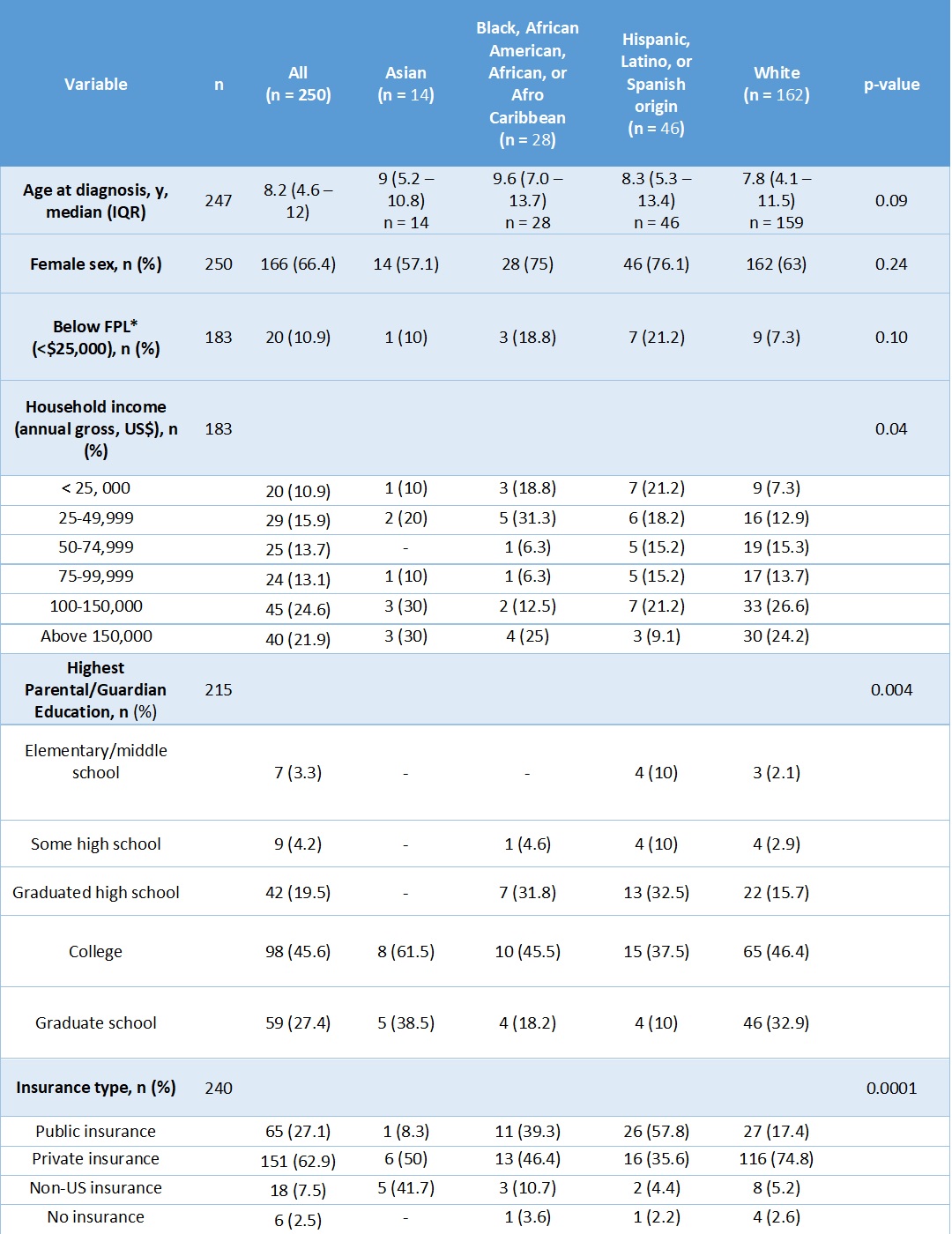 Table 1: Baseline demographic characteristics by race and ethnicity
Table 1: Baseline demographic characteristics by race and ethnicity
*FPL = below the federal poverty limit (FPL) for a family of 4 in 2019, which was approximately $25,750, per the US Census Bureau.
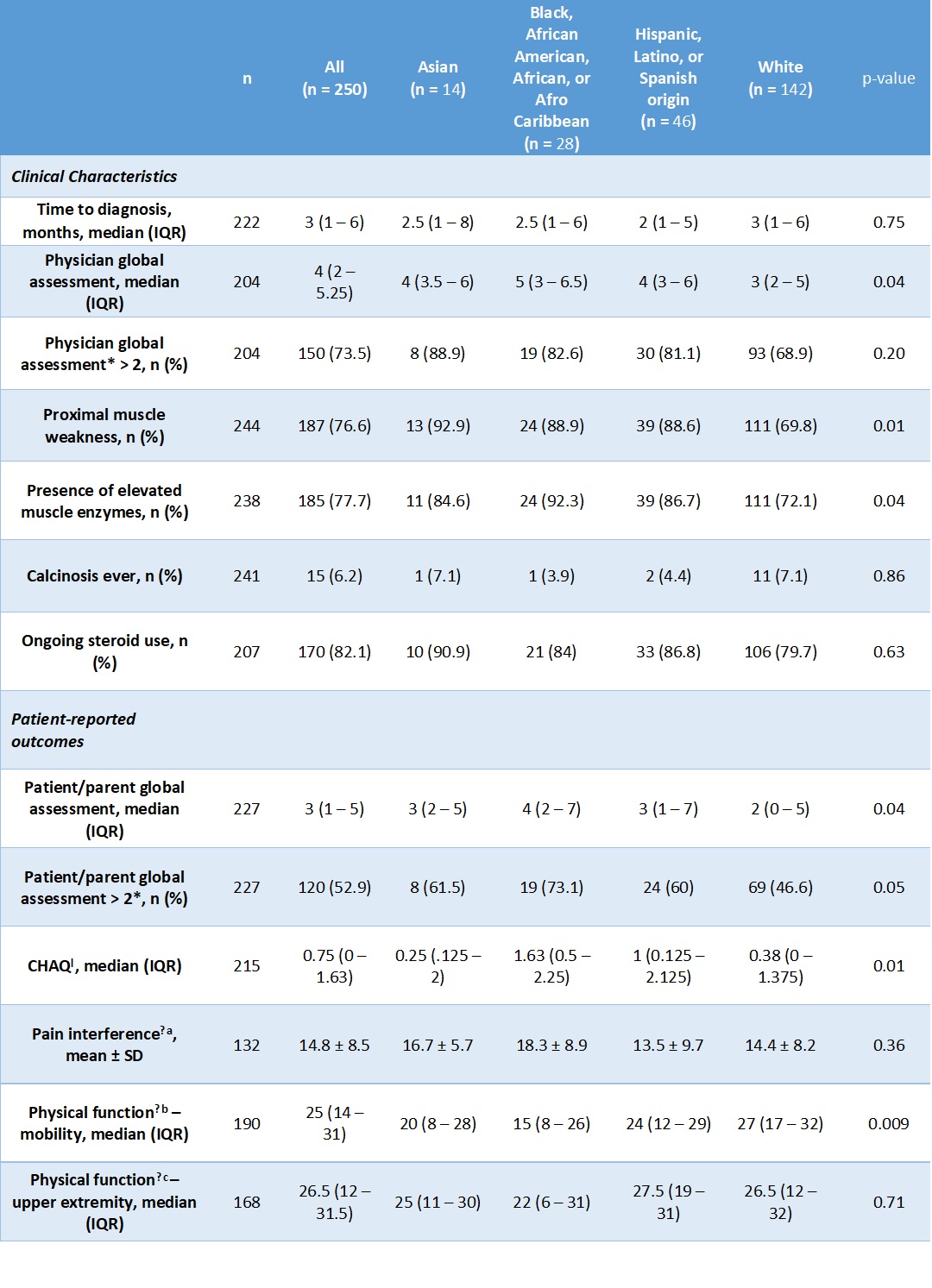 Table 2: Baseline disease characteristics and patient-reported outcomes.
Table 2: Baseline disease characteristics and patient-reported outcomes.
*A physician global assessment (PhGA) or patient/parent global assessment (PGA) score greater than 2 indicates worse disease activity.
ƚ CHAQ = Childhood Health Assessment Questionnaire; score range of 0 to 3, with higher scores indicating worse disease activity
ǂ Patient-Reported Outcomes Measurement Information System (PROMIS) measures
a. Pain Interference: patient-reported measurement of how much pain associated with activity impacts daily life, with a score range of 0 to 32, with higher scores indicating worse disease activity
b. Physical function – mobility: patient-reported measurement of mobility, with a score range of 0 to 32, with higher scores indicating worse disease activity
c. Physical function – upper extremity: patient-reported measurement of degree of limitation of physical function of the upper extremities, with a score range of 0 to 32, with higher scores indicating worse disease activity
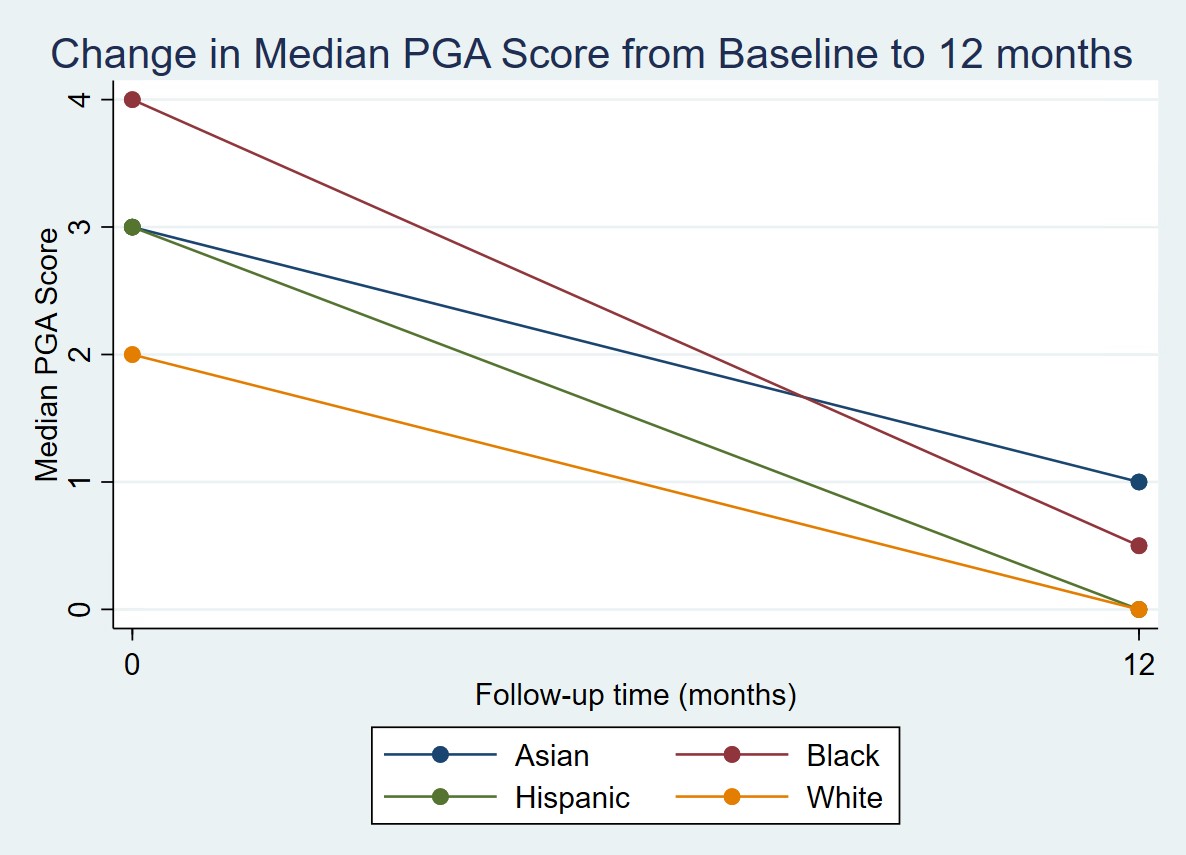 Figure 1: Change in median PGA score from baseline to 12 months by race and ethnicity
Figure 1: Change in median PGA score from baseline to 12 months by race and ethnicity
Disclosures: R. Olveda, None; J. Neely, None; S. Kim, None.
Background/Purpose: Previous studies in juvenile dermatomyositis (JDM) have shown that patients from minoritized ethnicities and those with lower family income are more likely to have worse clinical outcomes. Patient-reported outcome measures (PROs) are valuable indicators of disease activity but are less well-studied in JDM. This study aimed to investigate differences in clinical outcomes and PROs based on race and ethnicity at baseline and at 12 months, in a cohort of patients enrolled in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry.
Methods: This was a retrospective cohort study using data from children diagnosed with JDM and enrolled into the CARRA Registry between February 2018 and November 2021, for whom data regarding race and ethnicity were available. The primary predictor was self-identified race and/or ethnicity. The primary outcome was the Patient/Parent Global Assessment Score (PGA) as a dichotomous variable of score < /= 2 (lower disease activity) or > 2 (higher disease activity). Secondary outcomes included proximal muscle weakness, elevated muscle enzymes, and PGA score at 12 months. Descriptive statistics were used to summarize each variable, and demographic and baseline disease activity measures were evaluated across racial and ethnic categories using Chi-squared and Kruskal-Wallis tests. Demographic variables with significant association with race and ethnicity were included in a multivariate model analyzing PGA score greater than 2 at baseline.
Results: 250 participants identified as Asian (5.6%), Black (11.2%), Hispanic (18.4%), or White (64.8%). Demographic characteristics are listed in Table 1. A higher proportion of Black and Hispanic participants were in the lowest income brackets (p = 0.04) (Table 1). At baseline, Asian, Black, and Hispanic participants were more likely to have proximal muscle weakness (p = 0.01) and elevated muscle enzymes compared to White participants (p = 0.04) (Table 2). Black participants had 3.1 times the odds of having a PGA > 2 at baseline compared to White participants (95% CI 1.2 to 7.8, p = 0.02). After adjusting for age at diagnosis, income, education, and insurance status, this association was no longer statistically significant. White participants tended to have lower PGA scores at baseline and at 12 months compared to Black and Asian participants. However, PGA score at 12 months and change in PGA score over 1 year were not significantly different between racial and ethnic groups (Figure 1).
Conclusion: In JDM participants enrolled in the CARRA Registry, Black and Hispanic participants tended to have lower income and education levels. While Black participants had a higher odds of having worse PGA at baseline, this association diminished after adjusting for age at diagnosis, income, education, and insurance status. Our study was limited by a smaller proportion of participants from minoritized racial and ethnic groups. More research is needed to determine how patient outcomes are influenced by social determinants of health, including income, education, and the effects of systemic racism, in order to inform future interventions to improve health disparities.
 Table 1: Baseline demographic characteristics by race and ethnicity
Table 1: Baseline demographic characteristics by race and ethnicity*FPL = below the federal poverty limit (FPL) for a family of 4 in 2019, which was approximately $25,750, per the US Census Bureau.
 Table 2: Baseline disease characteristics and patient-reported outcomes.
Table 2: Baseline disease characteristics and patient-reported outcomes.*A physician global assessment (PhGA) or patient/parent global assessment (PGA) score greater than 2 indicates worse disease activity.
ƚ CHAQ = Childhood Health Assessment Questionnaire; score range of 0 to 3, with higher scores indicating worse disease activity
ǂ Patient-Reported Outcomes Measurement Information System (PROMIS) measures
a. Pain Interference: patient-reported measurement of how much pain associated with activity impacts daily life, with a score range of 0 to 32, with higher scores indicating worse disease activity
b. Physical function – mobility: patient-reported measurement of mobility, with a score range of 0 to 32, with higher scores indicating worse disease activity
c. Physical function – upper extremity: patient-reported measurement of degree of limitation of physical function of the upper extremities, with a score range of 0 to 32, with higher scores indicating worse disease activity
 Figure 1: Change in median PGA score from baseline to 12 months by race and ethnicity
Figure 1: Change in median PGA score from baseline to 12 months by race and ethnicityDisclosures: R. Olveda, None; J. Neely, None; S. Kim, None.

