Back
Poster Session C
Rheumatoid arthritis (RA)
Session: (1387–1416) RA – Diagnosis, Manifestations, and Outcomes Poster III
1406: Integrative Multi-omic Phenotyping in Blood Identifies Molecular Signatures and Candidate Biomarkers of ACPA-negative Rheumatoid Arthritis
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- BH
Benjamin Hur, PhD
Mayo Clinic
Rochester, MN, United States
Abstract Poster Presenter(s)
Benjamin Hur1, Kevin Cunningham2, John Davis1 and Jaeyun Sung1, 1Mayo Clinic, Rochester, MN, 2University of Minnesota, Rochester, MN
Background/Purpose: ACPA detection assays are often used for RA diagnosis due to their high specificity ( >90%). However, current ACPA assays (e.g., anti-CCP2 ELISA) have far lower sensitivity (30–60%), and thus patients can still be clinically diagnosed with RA while testing negative for ACPA (or otherwise known as the "ACPA-negative" RA subgroup). This can make the diagnosis of RA quite challenging for early-stage RA patients, resulting in delays in starting therapeutic interventions. In addition, ACPA-negative RA patients are about 40% of the RA patient population and can show different disease course compared to ACPA-positive patients. To better understand the biomolecular characteristics of ACPA-negative RA given its clinical significance, we performed an integrative multi-omic phenotyping analysis on blood (plasma) samples from RA patients of two different subgroups (i.e., ACPA-negative, ACPA-positive) and healthy controls. Our aims were to identify features specific to ACPA-negative RA and potential biomarkers for ACPA-negative RA diagnostics.
Methods: Global proteomic, metabolomic, and autoantibody profiling were performed on plasma samples from ACPA-negative RA patients (n = 40), sex-/age-matched ACPA-positive RA patients (n = 40), and healthy controls (n = 40) (Fig. 1). A linear regression model was used to identify multi-omics features associated with ACPA-negative RA. ElasticNet was used to infer a multiplex network (from the multi-omics data), which characterizes associations among multi-omic features and clinical conditions in a single analytical framework (Fig. 2A, Steps 1–2). A machine-learning strategy using a network-based feature selection scheme was developed for RA subgroup classification (Fig. 2A, Steps 3–5).
Results: We identified various plasma proteins, metabolites, or autoantibodies associated with RA subgroups. In particular, compared to healthy controls, we identified 23 upregulated and 49 downregulated plasma proteins in ACPA-negative RA; and 25 upregulated and 32 downregulated plasma proteins in ACPA-positive RA. Proteins that were differentially abundant in ACPA-negative RA were found to be enriched in innate immune response and metabolic processes, while proteins that were differentially abundant in ACPA-positive RA were found to be enriched in chemotaxis- and cell migration-related functions. Interestingly, the identified differentially abundant metabolites and autoantibodies were found to support our proteomics results. Finally, our network-based machine-learning classification scheme was able to distinguish ACPA-negative RA patients from healthy controls with 90.0% accuracy, and classify RA patients (of both subgroups) and healthy controls with 86.6% accuracy (Fig. 2B).
Conclusion: We demonstrate the first simultaneous proteomic, metabolomic, and autoantibody phenotyping in the blood of patients with ACPA-negative RA. Our computational analysis identifies multi-omics features that may explain the similarity and differences in biomolecular processes between the ACPA-negative and ACPA-positive subgroups of RA; and suggests potential biomarkers with clinical applicability in blood-based RA diagnostics.
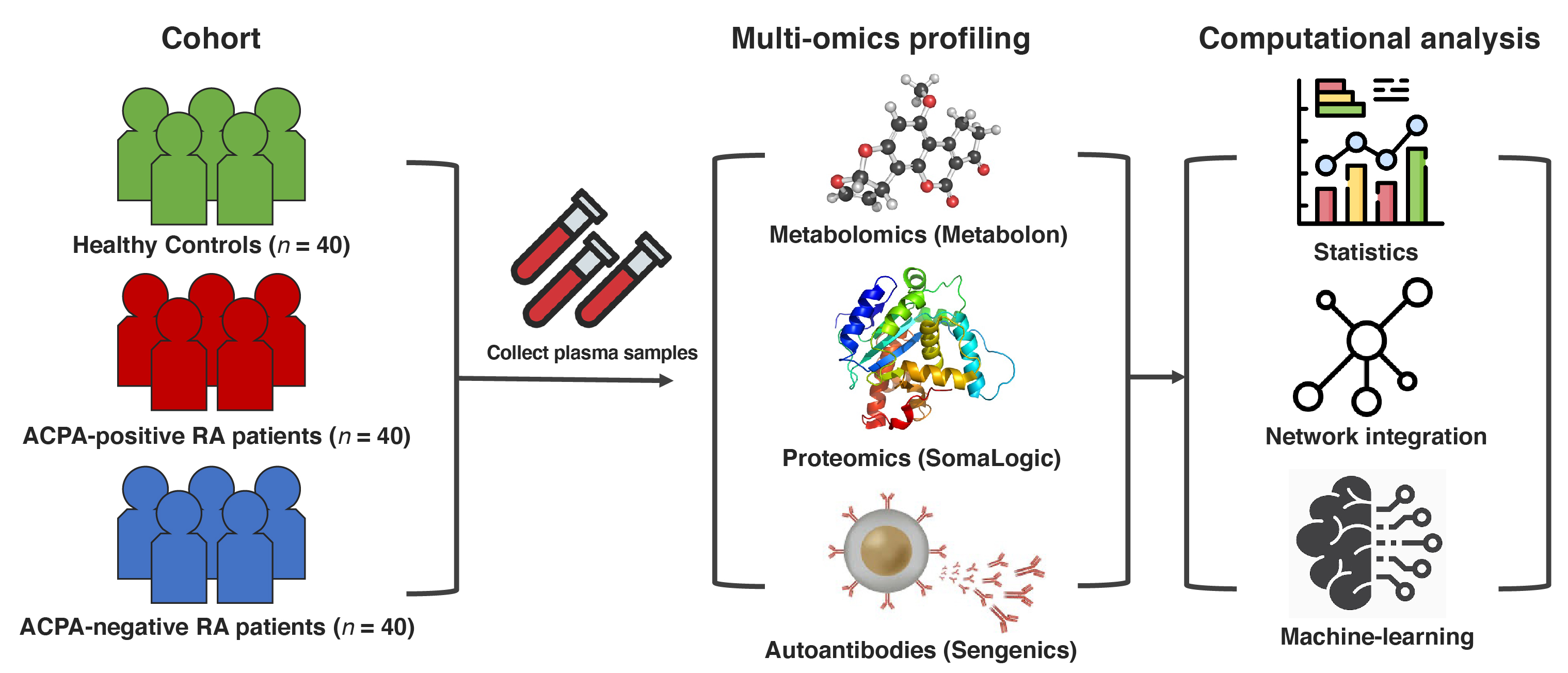 Figure 1. Study design overview and data analysis strategy. Plasma samples were collected from ACPA-negative RA patients (n = 40), sex-/age-matched ACPA-positive RA patients (n = 40), and healthy controls (n = 40). Global proteomic, metabolomic, and autoantibody phenotyping were performed on the plasma samples. On the ensuing multi-omics data, computational analyses were performed to identify biomolecular features specific to ACPA-negative RA and potential computational biomarkers for ACPA-negative RA diagnostics.
Figure 1. Study design overview and data analysis strategy. Plasma samples were collected from ACPA-negative RA patients (n = 40), sex-/age-matched ACPA-positive RA patients (n = 40), and healthy controls (n = 40). Global proteomic, metabolomic, and autoantibody phenotyping were performed on the plasma samples. On the ensuing multi-omics data, computational analyses were performed to identify biomolecular features specific to ACPA-negative RA and potential computational biomarkers for ACPA-negative RA diagnostics.
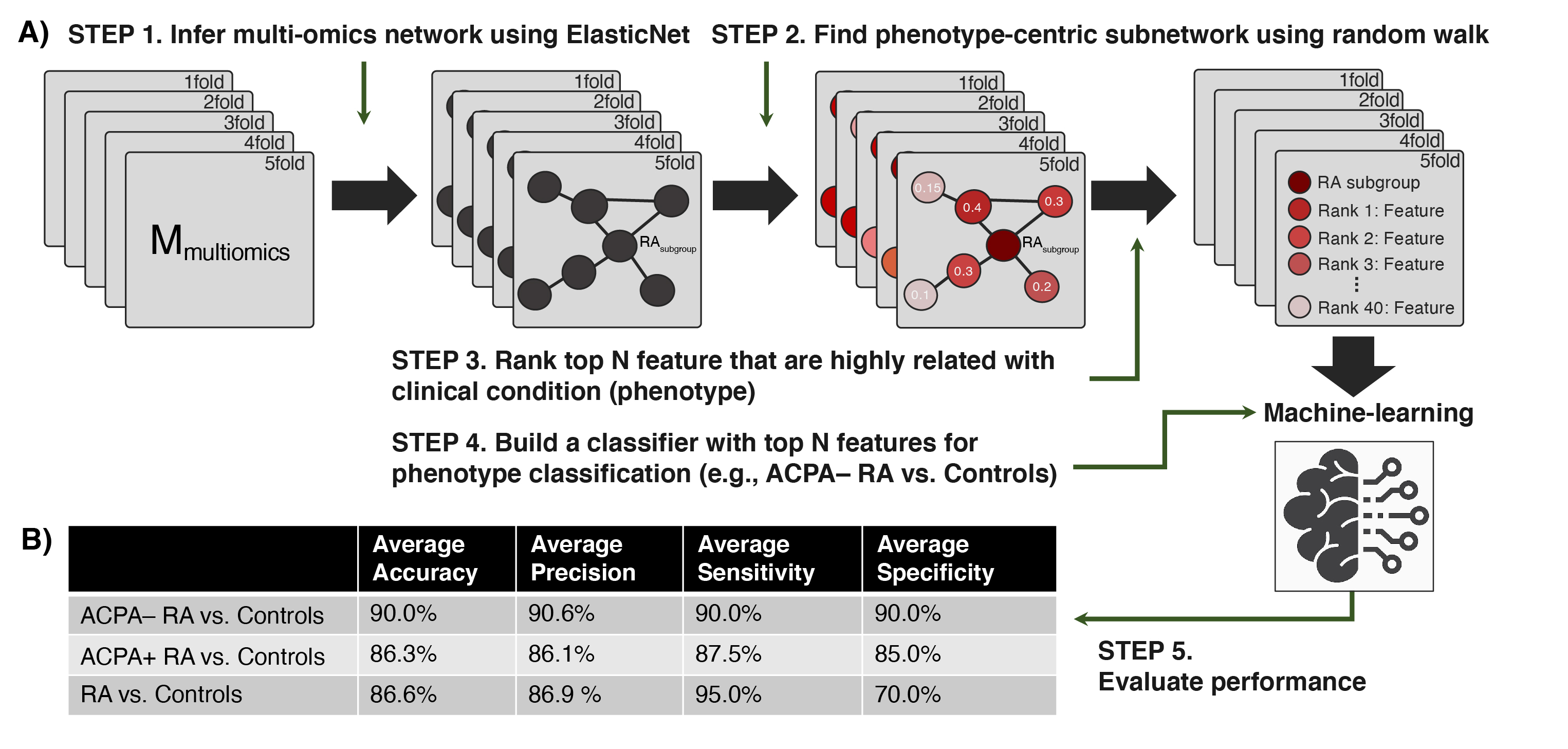 Figure 2. Performance of our network-driven, integrative multi-omics biomarker discovery approach was evaluated in 5-fold cross-validation. (A) A machine-learning framework that (i) infers a multiplex network from multi-omics data, (ii) identifies a subset of the multiplex network (a subnetwork) that includes only the features most associated with an RA subgroup, and (iii) uses the top N features to design classifiers for RA. (B) Average accuracy, precision, sensitivity, and specificity in each classification scenario. Of note, our machine-learning strategy obtained 90.0% (or slightly higher) results in all four metrics when distinguishing ACPA-negative RA from controls.
Figure 2. Performance of our network-driven, integrative multi-omics biomarker discovery approach was evaluated in 5-fold cross-validation. (A) A machine-learning framework that (i) infers a multiplex network from multi-omics data, (ii) identifies a subset of the multiplex network (a subnetwork) that includes only the features most associated with an RA subgroup, and (iii) uses the top N features to design classifiers for RA. (B) Average accuracy, precision, sensitivity, and specificity in each classification scenario. Of note, our machine-learning strategy obtained 90.0% (or slightly higher) results in all four metrics when distinguishing ACPA-negative RA from controls.
Disclosures: B. Hur, None; K. Cunningham, None; J. Davis, Pfizer; J. Sung, None.
Background/Purpose: ACPA detection assays are often used for RA diagnosis due to their high specificity ( >90%). However, current ACPA assays (e.g., anti-CCP2 ELISA) have far lower sensitivity (30–60%), and thus patients can still be clinically diagnosed with RA while testing negative for ACPA (or otherwise known as the "ACPA-negative" RA subgroup). This can make the diagnosis of RA quite challenging for early-stage RA patients, resulting in delays in starting therapeutic interventions. In addition, ACPA-negative RA patients are about 40% of the RA patient population and can show different disease course compared to ACPA-positive patients. To better understand the biomolecular characteristics of ACPA-negative RA given its clinical significance, we performed an integrative multi-omic phenotyping analysis on blood (plasma) samples from RA patients of two different subgroups (i.e., ACPA-negative, ACPA-positive) and healthy controls. Our aims were to identify features specific to ACPA-negative RA and potential biomarkers for ACPA-negative RA diagnostics.
Methods: Global proteomic, metabolomic, and autoantibody profiling were performed on plasma samples from ACPA-negative RA patients (n = 40), sex-/age-matched ACPA-positive RA patients (n = 40), and healthy controls (n = 40) (Fig. 1). A linear regression model was used to identify multi-omics features associated with ACPA-negative RA. ElasticNet was used to infer a multiplex network (from the multi-omics data), which characterizes associations among multi-omic features and clinical conditions in a single analytical framework (Fig. 2A, Steps 1–2). A machine-learning strategy using a network-based feature selection scheme was developed for RA subgroup classification (Fig. 2A, Steps 3–5).
Results: We identified various plasma proteins, metabolites, or autoantibodies associated with RA subgroups. In particular, compared to healthy controls, we identified 23 upregulated and 49 downregulated plasma proteins in ACPA-negative RA; and 25 upregulated and 32 downregulated plasma proteins in ACPA-positive RA. Proteins that were differentially abundant in ACPA-negative RA were found to be enriched in innate immune response and metabolic processes, while proteins that were differentially abundant in ACPA-positive RA were found to be enriched in chemotaxis- and cell migration-related functions. Interestingly, the identified differentially abundant metabolites and autoantibodies were found to support our proteomics results. Finally, our network-based machine-learning classification scheme was able to distinguish ACPA-negative RA patients from healthy controls with 90.0% accuracy, and classify RA patients (of both subgroups) and healthy controls with 86.6% accuracy (Fig. 2B).
Conclusion: We demonstrate the first simultaneous proteomic, metabolomic, and autoantibody phenotyping in the blood of patients with ACPA-negative RA. Our computational analysis identifies multi-omics features that may explain the similarity and differences in biomolecular processes between the ACPA-negative and ACPA-positive subgroups of RA; and suggests potential biomarkers with clinical applicability in blood-based RA diagnostics.
 Figure 1. Study design overview and data analysis strategy. Plasma samples were collected from ACPA-negative RA patients (n = 40), sex-/age-matched ACPA-positive RA patients (n = 40), and healthy controls (n = 40). Global proteomic, metabolomic, and autoantibody phenotyping were performed on the plasma samples. On the ensuing multi-omics data, computational analyses were performed to identify biomolecular features specific to ACPA-negative RA and potential computational biomarkers for ACPA-negative RA diagnostics.
Figure 1. Study design overview and data analysis strategy. Plasma samples were collected from ACPA-negative RA patients (n = 40), sex-/age-matched ACPA-positive RA patients (n = 40), and healthy controls (n = 40). Global proteomic, metabolomic, and autoantibody phenotyping were performed on the plasma samples. On the ensuing multi-omics data, computational analyses were performed to identify biomolecular features specific to ACPA-negative RA and potential computational biomarkers for ACPA-negative RA diagnostics. Figure 2. Performance of our network-driven, integrative multi-omics biomarker discovery approach was evaluated in 5-fold cross-validation. (A) A machine-learning framework that (i) infers a multiplex network from multi-omics data, (ii) identifies a subset of the multiplex network (a subnetwork) that includes only the features most associated with an RA subgroup, and (iii) uses the top N features to design classifiers for RA. (B) Average accuracy, precision, sensitivity, and specificity in each classification scenario. Of note, our machine-learning strategy obtained 90.0% (or slightly higher) results in all four metrics when distinguishing ACPA-negative RA from controls.
Figure 2. Performance of our network-driven, integrative multi-omics biomarker discovery approach was evaluated in 5-fold cross-validation. (A) A machine-learning framework that (i) infers a multiplex network from multi-omics data, (ii) identifies a subset of the multiplex network (a subnetwork) that includes only the features most associated with an RA subgroup, and (iii) uses the top N features to design classifiers for RA. (B) Average accuracy, precision, sensitivity, and specificity in each classification scenario. Of note, our machine-learning strategy obtained 90.0% (or slightly higher) results in all four metrics when distinguishing ACPA-negative RA from controls.Disclosures: B. Hur, None; K. Cunningham, None; J. Davis, Pfizer; J. Sung, None.

