Back
Poster Session C
Epidemiology, health policy and outcomes
Session: (1186–1214) Epidemiology and Public Health Poster II
1200: Vitamin D and Marine n-3 Fatty Acid Supplementation for Prevention of Autoimmune Disease in the VITAL Randomized Controlled Trial: Outcomes over 7 Years
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Karen Costenbader, MD, MPH
Brigham and Women's Hospital/ Harvard Medical School
BOSTON, MA, United States
Abstract Poster Presenter(s)
Karen Costenbader1, Nancy R Cook2, I-Min Lee2, Jill Hahn3, Joseph Walter1, Vadim Bubes1, Gregory Kotler1, Nicole Yang1, Sonia Friedman1, Erik K Alexander1 and JoAnn E Manson2, 1Brigham and Women's Hospital, Boston, MA, 2Harvard TH Chan School of Public Health, Brigham and Women's Hospital, Boston, MA, 3Brigham and Womens' Hospital, Boston, MA
Background/Purpose: Strong biologic rationale supports potential effects of both vitamin D and of marine omega-3 (n3) fatty acids for autoimmune disease prevention. Within the randomized double-blind, placebo-controlled VITamin D and OmegA-3 TriaL (VITAL), we tested the effects of these supplements on autoimmune disease incidence. We previously reported results after 5.3 years of randomized follow-up showing overall protective effects for vitamin D on incidence of all autoimmune diseases (hazard ratio [HR] 0.78, 95% confidence interval [CI] 0.61-0.99) and suggestive effect for n-3 fatty acids (HR 0.85, 95%CI 0.67-1.08) (Hahn J et al, BMJ, 2022). We now test the effects of these 2 supplements with 2 more years of post-intervention follow-up in VITAL to determine if the effects were sustained after the intervention ended.
Methods: VITAL enrolled and randomized men and women (age >50 and >55 years, respectively) in a two-by-two factorial design to receive vitamin D3 (2000 IU/d) and/or n3 fatty acids (1000 mg/d) or placebo and followed them for median of 5.3 years. Here, we followed surviving and willing participants (85%) for another 2 years of observation to assess for sustained effects. Incident diagnoses of autoimmune diseases were reported by participants annually and confirmed by medical record review by expert physicians using classification criteria. The primary endpoint was all confirmed incident autoimmune diseases, including rheumatoid arthritis (RA), polymyalgia rheumatica (PMR), autoimmune thyroid disease, psoriasis, and others. Pre-specified secondary endpoints included confirmed and probable cases, and individual autoimmune diseases. HRs for incident autoimmune diseases were calculated using Cox regression models.
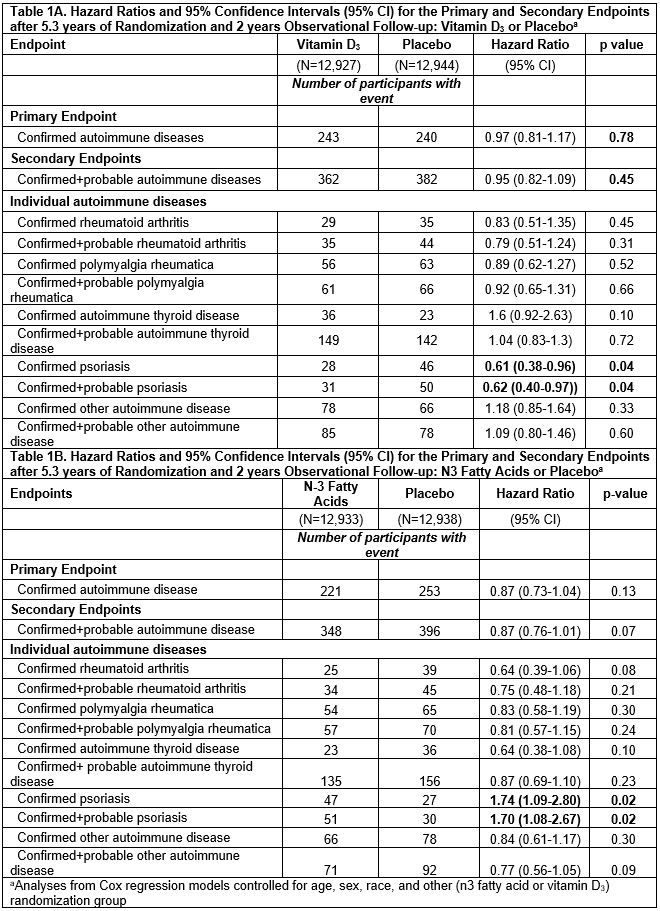
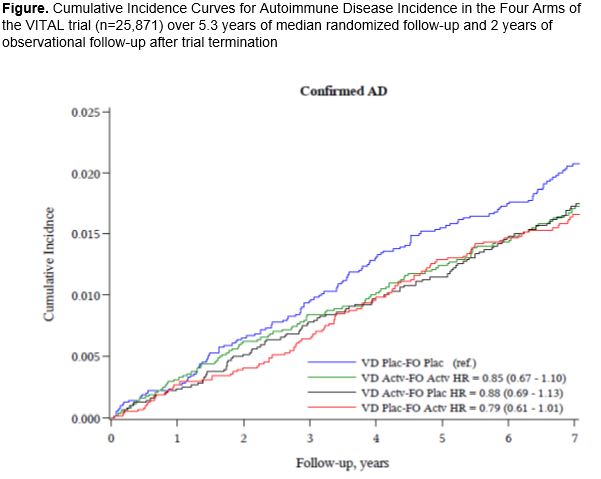
Results: 25,871 participants were randomized: 71% self-reported as non-Hispanic Whites, 20% Black, and 9% other racial/ethnic groups; 51% women; mean age 67.1 years. During median follow-up of 7.3 years, confirmed autoimmune disease was diagnosed in 234 participants in the vitamin D3 group and 240 in the placebo group, HR 0.97 (0.81-1.17). (Table 1A) Confirmed autoimmune disease was diagnosed in 221 participants in the n3 fatty acids group and 253 in the placebo group, HR 0.87 (0.73-1.04). For vitamin D, HRs were RA 0.83 (0.51-1.35), PMR 0.89 (0.62-1.27) and psoriasis 0.61 (0.38-0.96). For n3 fatty acids, HRs trended toward reduction for RA 0.64 (0.39-1.06) and autoimmune thyroid disease 0.64 (0.38-1.08), but psoriasis was increased 1.72 (1.09-2.80). In the 2x2 factorial groups, all intervention arms continued to have more reduced incidence of new autoimmune diseases than did the placebo/placebo arm. (Figure) The effect of vitamin D on autoimmune disease incidence was stronger among those with body mass index (BMI) < 25 kg/m2 compared to ≥ 25 kg/m2 (p-interaction 0.01).
Conclusion: 2000 IU/day vitamin D led to significantly reduced incidence of autoimmune diseases in this trial over 5.3 years, but after trial termination, protective effects dissipated and were no longer significant. By contrast, 1000 mg/day of n3 fatty acids led to borderline reduction of autoimmune disease incidence over 5.3 years, with minimal change after 2 years of post-trial follow-up.
Disclosures: K. Costenbader, Eli Lilly, Janssen, Amgen, AstraZeneca Pharmaceuticals LP, GlaxoSmithKline(GSK), Gilead, Exagen, Neutrolis, Cel-Sci, Alkermes; N. Cook, None; I. Lee, None; J. Hahn, None; J. Walter, None; V. Bubes, None; G. Kotler, None; N. Yang, None; S. Friedman, None; E. Alexander, None; J. Manson, None.
Background/Purpose: Strong biologic rationale supports potential effects of both vitamin D and of marine omega-3 (n3) fatty acids for autoimmune disease prevention. Within the randomized double-blind, placebo-controlled VITamin D and OmegA-3 TriaL (VITAL), we tested the effects of these supplements on autoimmune disease incidence. We previously reported results after 5.3 years of randomized follow-up showing overall protective effects for vitamin D on incidence of all autoimmune diseases (hazard ratio [HR] 0.78, 95% confidence interval [CI] 0.61-0.99) and suggestive effect for n-3 fatty acids (HR 0.85, 95%CI 0.67-1.08) (Hahn J et al, BMJ, 2022). We now test the effects of these 2 supplements with 2 more years of post-intervention follow-up in VITAL to determine if the effects were sustained after the intervention ended.
Methods: VITAL enrolled and randomized men and women (age >50 and >55 years, respectively) in a two-by-two factorial design to receive vitamin D3 (2000 IU/d) and/or n3 fatty acids (1000 mg/d) or placebo and followed them for median of 5.3 years. Here, we followed surviving and willing participants (85%) for another 2 years of observation to assess for sustained effects. Incident diagnoses of autoimmune diseases were reported by participants annually and confirmed by medical record review by expert physicians using classification criteria. The primary endpoint was all confirmed incident autoimmune diseases, including rheumatoid arthritis (RA), polymyalgia rheumatica (PMR), autoimmune thyroid disease, psoriasis, and others. Pre-specified secondary endpoints included confirmed and probable cases, and individual autoimmune diseases. HRs for incident autoimmune diseases were calculated using Cox regression models.
Results: 25,871 participants were randomized: 71% self-reported as non-Hispanic Whites, 20% Black, and 9% other racial/ethnic groups; 51% women; mean age 67.1 years. During median follow-up of 7.3 years, confirmed autoimmune disease was diagnosed in 234 participants in the vitamin D3 group and 240 in the placebo group, HR 0.97 (0.81-1.17). (Table 1A) Confirmed autoimmune disease was diagnosed in 221 participants in the n3 fatty acids group and 253 in the placebo group, HR 0.87 (0.73-1.04). For vitamin D, HRs were RA 0.83 (0.51-1.35), PMR 0.89 (0.62-1.27) and psoriasis 0.61 (0.38-0.96). For n3 fatty acids, HRs trended toward reduction for RA 0.64 (0.39-1.06) and autoimmune thyroid disease 0.64 (0.38-1.08), but psoriasis was increased 1.72 (1.09-2.80). In the 2x2 factorial groups, all intervention arms continued to have more reduced incidence of new autoimmune diseases than did the placebo/placebo arm. (Figure) The effect of vitamin D on autoimmune disease incidence was stronger among those with body mass index (BMI) < 25 kg/m2 compared to ≥ 25 kg/m2 (p-interaction 0.01).
Conclusion: 2000 IU/day vitamin D led to significantly reduced incidence of autoimmune diseases in this trial over 5.3 years, but after trial termination, protective effects dissipated and were no longer significant. By contrast, 1000 mg/day of n3 fatty acids led to borderline reduction of autoimmune disease incidence over 5.3 years, with minimal change after 2 years of post-trial follow-up.


Disclosures: K. Costenbader, Eli Lilly, Janssen, Amgen, AstraZeneca Pharmaceuticals LP, GlaxoSmithKline(GSK), Gilead, Exagen, Neutrolis, Cel-Sci, Alkermes; N. Cook, None; I. Lee, None; J. Hahn, None; J. Walter, None; V. Bubes, None; G. Kotler, None; N. Yang, None; S. Friedman, None; E. Alexander, None; J. Manson, None.

