Back
Ignite Talk
Session: Ignite Session 6A
1298: Pediatric Rheumatology Care and Outcomes Improvement Network Demonstrates Outcome Improvement for Children with Juvenile Idiopathic Arthritis
Sunday, November 13, 2022
2:10 PM – 2:15 PM Eastern Time
Location: Northern Liberties Stage
- CB
Catherine Bingham, MD
Penn State Children's Hospital
Hershey, PA, United StatesDisclosure: Disclosure information not submitted.
Ignite Speaker(s)
Catherine Bingham1, Julia Harris2, Sheetal Vora3, Mileka Gilbert4, Cagri Yildirim-Toruner5, Kerry Ferraro6, Tingting Qiu7, Jon Burnham8, Michelle Batthish9, Beth Gottlieb10, Daniel Lovell7, Ronald Laxer11, Tzielan Lee12, Danielle Bullock13, Charles H Spencer14, Jennifer Weiss15, Melissa Hazen16, Edward Oberle17, Melissa Mannion18, Nancy Pan19, Michael Shishov20, Danielle Fair21, Mary Toth22, Kendra Wiegand7 and Esi Morgan23, 1Penn State Children's Hospital, Hershey, PA, 2Children's Mercy Kansas City, Overland Park, KS, 3Atrium Health Levine Children's, Charlotte, NC, 4Medical University of South Carolina, Charleston, SC, 5Texas Children's Hospital/ Baylor College of Medicine, Houston, TX, 6Pediatric Rheumatology Care and Outcomes Improvement Network, Philadelphia, 7Cincinnati Children's Hospital Medical Center, Cincinnati, OH, 8Children's Hospital of Philadelphia, Philadelphia, PA, 9McMaster University, Hamilton, ON, Canada, 10Cohen Children's Medical Center, Lake Success, NY, 11Division of Rheumatology, The Hospital for Sick Children; Child Health Evaluative Services, SickKids Research Institute; Department of Paediatrics, University of Toronto, Toronto, ON, Canada, 12Stanford University School of Medicine, Palo Alto, CA, 13University of Minnesota, Minneapolis, MN, 14Division of Rheumatology, Nationwide Children’s Hospital and Department of Pediatrics, The Ohio State University, Columbus, MS, 15Hackensack University Medical Center, Hackensack, NJ, 16Boston Children's Hospital, Boston, MA, 17Division of Rheumatology, Nationwide Children's Hospital and Department of Pediatrics, The Ohio State University, Columbus, OH, 18University of Alabama at Birmingham, Birmingham, AL, 19Hospital for Special Surgery, New York, NY, 20Phoenix Children's Hospital, Phoenix, AZ, 21Medical College of Wisconsin/Children's Wisconsin, Wauwatosa, WI, 22Nemours Foundation, Orlando, FL, 23Seattle Children's Hospital, Seattle, WA
Background/Purpose: Pediatric Rheumatology Care and Outcomes Improvement Network (PR-COIN) is a Learning Health Network designed to improve outcomes of care for children with juvenile idiopathic arthritis (JIA). Currently, 23 pediatric rheumatology centers in North America participate, and a subset are currently entering data into a network Registry with a goal to report performance on process and outcome quality measures (QMs) to drive improved outcomes.
Methods: PR-COIN centers learn and use established quality improvement (QI) methodology to conduct improvement work. Since 2011, participating sites have entered data from JIA clinical encounters into a shared Registry. Performance on QMs is reported on statistical process control charts for both site-specific and aggregate data to identify significant change in performance, or special cause variation. Disease specific outcome measures include the clinical Juvenile Arthritis Disease Activity Score (cJADAS10), clinically inactive disease (CID), active joint count (AJC), and the time to inactive disease or low disease activity (LDA). Patient-reported outcomes measures (PROMs) include optimal physical function, low or no pain as defined as arthritis pain score < /=3, and patient global assessment of overall well-being (PtGA).
Results: Recent PR-COIN performance data includes 2043 children with JIA, and a total of >8200 children with JIA have been enrolled in the PR-COIN Registry since 2011. Disease activity outcomes have improved over time including mean AJC, mean cJADAS10, percent of patients with oligoarticular or polyarticular JIA who have inactive disease or LDA by cJADAS10, and percent of patients with CID (excluding inflammatory markers) (Figure 1). PROMs have also improved including mean PtGA, percent of patients with PtGA < /= 2, percent of patients with optimal physical function, and percent of patients with low or no pain (Figure 2). Figure 3 illustrates the control chart showing improvement in percentage of patients with PtGA < /=2 from 67% to 75%. Recent data reflects approximately 37% of the patients enrolled in the PR-COIN Registry and currently receiving pediatric rheumatology care at PR-COIN sites.
Conclusion: PR-COIN, a multi-center learning network implementing QI science that utilizes a shared Registry to report QMs and identify best practices, has demonstrated success in improving outcomes for children with JIA. Ongoing analysis of outcome performance will continue as more patients are included in the Registry. Data quality efforts are underway to ensure complete and representative data.
.jpg) Figure 1: Y axis represents mean score (disease activity by cJADAS10 and AJC) and percent of patients who achieve the measure (inactive or LDA, and Clinical Inactive Disease).
Figure 1: Y axis represents mean score (disease activity by cJADAS10 and AJC) and percent of patients who achieve the measure (inactive or LDA, and Clinical Inactive Disease).
.jpg) Figure 2: Y axis represents mean PtGA score and percent of patients who achieve the measure (optimal physical function, no or mild pain, and PtGA
Figure 2: Y axis represents mean PtGA score and percent of patients who achieve the measure (optimal physical function, no or mild pain, and PtGA
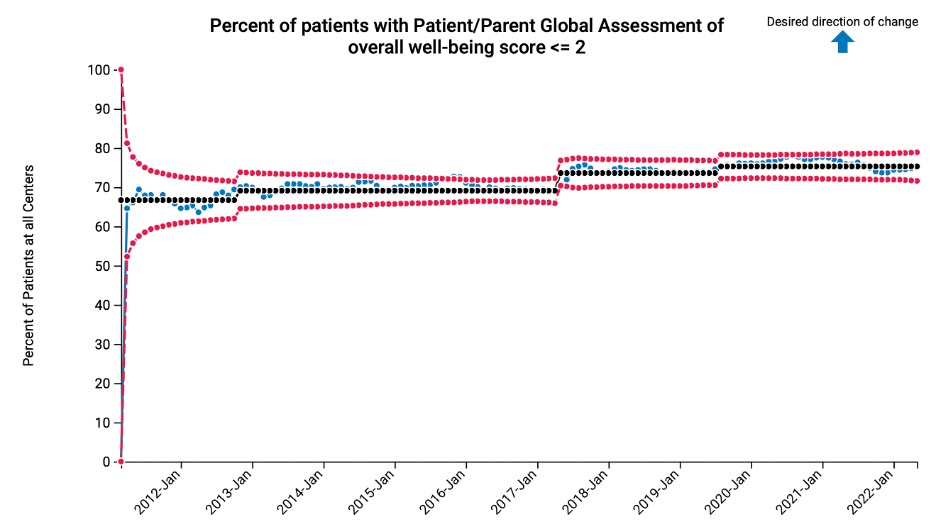 Figure 3: Blue dotted line=PR-COIN aggregate performance; Black dotted line=mean; Red dotted lines=control limits (+/-3 standard error of mean)
Figure 3: Blue dotted line=PR-COIN aggregate performance; Black dotted line=mean; Red dotted lines=control limits (+/-3 standard error of mean)
Disclosures: C. Bingham, None; J. Harris, None; S. Vora, None; M. Gilbert, None; C. Yildirim-Toruner, None; K. Ferraro, None; T. Qiu, None; J. Burnham, None; M. Batthish, None; B. Gottlieb, None; D. Lovell, AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKlein(GSK), Novartis, UCB, Bristol-Myers Squibb(BMS), Pfizer, Janssen, NIH/NIAMS, NIH/NICHD, Roche; R. Laxer, Eli Lilly, Novartis, sanofi, sobi; T. Lee, None; D. Bullock, None; C. Spencer, None; J. Weiss, None; M. Hazen, None; E. Oberle, None; M. Mannion, None; N. Pan, None; M. Shishov, None; D. Fair, None; M. Toth, None; K. Wiegand, None; E. Morgan, None.
Background/Purpose: Pediatric Rheumatology Care and Outcomes Improvement Network (PR-COIN) is a Learning Health Network designed to improve outcomes of care for children with juvenile idiopathic arthritis (JIA). Currently, 23 pediatric rheumatology centers in North America participate, and a subset are currently entering data into a network Registry with a goal to report performance on process and outcome quality measures (QMs) to drive improved outcomes.
Methods: PR-COIN centers learn and use established quality improvement (QI) methodology to conduct improvement work. Since 2011, participating sites have entered data from JIA clinical encounters into a shared Registry. Performance on QMs is reported on statistical process control charts for both site-specific and aggregate data to identify significant change in performance, or special cause variation. Disease specific outcome measures include the clinical Juvenile Arthritis Disease Activity Score (cJADAS10), clinically inactive disease (CID), active joint count (AJC), and the time to inactive disease or low disease activity (LDA). Patient-reported outcomes measures (PROMs) include optimal physical function, low or no pain as defined as arthritis pain score < /=3, and patient global assessment of overall well-being (PtGA).
Results: Recent PR-COIN performance data includes 2043 children with JIA, and a total of >8200 children with JIA have been enrolled in the PR-COIN Registry since 2011. Disease activity outcomes have improved over time including mean AJC, mean cJADAS10, percent of patients with oligoarticular or polyarticular JIA who have inactive disease or LDA by cJADAS10, and percent of patients with CID (excluding inflammatory markers) (Figure 1). PROMs have also improved including mean PtGA, percent of patients with PtGA < /= 2, percent of patients with optimal physical function, and percent of patients with low or no pain (Figure 2). Figure 3 illustrates the control chart showing improvement in percentage of patients with PtGA < /=2 from 67% to 75%. Recent data reflects approximately 37% of the patients enrolled in the PR-COIN Registry and currently receiving pediatric rheumatology care at PR-COIN sites.
Conclusion: PR-COIN, a multi-center learning network implementing QI science that utilizes a shared Registry to report QMs and identify best practices, has demonstrated success in improving outcomes for children with JIA. Ongoing analysis of outcome performance will continue as more patients are included in the Registry. Data quality efforts are underway to ensure complete and representative data.
.jpg) Figure 1: Y axis represents mean score (disease activity by cJADAS10 and AJC) and percent of patients who achieve the measure (inactive or LDA, and Clinical Inactive Disease).
Figure 1: Y axis represents mean score (disease activity by cJADAS10 and AJC) and percent of patients who achieve the measure (inactive or LDA, and Clinical Inactive Disease)..jpg) Figure 2: Y axis represents mean PtGA score and percent of patients who achieve the measure (optimal physical function, no or mild pain, and PtGA
Figure 2: Y axis represents mean PtGA score and percent of patients who achieve the measure (optimal physical function, no or mild pain, and PtGA  Figure 3: Blue dotted line=PR-COIN aggregate performance; Black dotted line=mean; Red dotted lines=control limits (+/-3 standard error of mean)
Figure 3: Blue dotted line=PR-COIN aggregate performance; Black dotted line=mean; Red dotted lines=control limits (+/-3 standard error of mean)Disclosures: C. Bingham, None; J. Harris, None; S. Vora, None; M. Gilbert, None; C. Yildirim-Toruner, None; K. Ferraro, None; T. Qiu, None; J. Burnham, None; M. Batthish, None; B. Gottlieb, None; D. Lovell, AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKlein(GSK), Novartis, UCB, Bristol-Myers Squibb(BMS), Pfizer, Janssen, NIH/NIAMS, NIH/NICHD, Roche; R. Laxer, Eli Lilly, Novartis, sanofi, sobi; T. Lee, None; D. Bullock, None; C. Spencer, None; J. Weiss, None; M. Hazen, None; E. Oberle, None; M. Mannion, None; N. Pan, None; M. Shishov, None; D. Fair, None; M. Toth, None; K. Wiegand, None; E. Morgan, None.

