Back
Abstract Session
Epidemiology, health policy and outcomes
Session: Abstracts: Measures and Measurement of Healthcare Quality (0570–0573)
0570: Clarifying Misbeliefs About Hydroxychloroquine (HCQ): Developing an Evidence-Based HCQ Benefits vs. Risk Decision Aid (HCQ-SAFE) Per Low Health Literacy Standards
Sunday, November 13, 2022
8:00 AM – 8:10 AM Eastern Time
Location: Room 121
- SG
Shivani Garg, MD, MS
University of Madison, School of Medicine and Public Health
Madison, WI, United States
Presenting Author(s)
Shivani Garg1, Sancia Ferguson2, Betty Chewning3, Shelby Gomez4, Jon Keevil5 and Christie Bartels6, 1University of Madison, School of Medicine and Public Health, Madison, WI, 2University of Wisconsin School of Medicine & Public Health, Madison, WI, 3University of Wisconsin, School of Pharmacy, Madison, WI, 4UW Health, Stoughton, WI, 5NA, Madison, WI, 6University of Wisconsin School of Medicine and Public Health, Madison, WI
Background/Purpose: Studies report ~83% of SLE patients discontinue hydroxychloroquine (HCQ) and many report suboptimal shared decision-making with their healthcare team. Moreover, patients report knowledge gaps on role of HCQ in improving survival in SLE, and inflated concerns about rare eye toxicity. In complex diseases, involving patients as partners to make therapy decisions can improve adherence and outcomes. Further, AHRQ calls for interventions meeting low health literacy standards. The objective was to describe the development and piloting of a decision aid (HCQ-SAFE) to facilitate shared decision making about HCQ's safety and efficacy.
Methods: Based on prior decision aid development, HCQ-SAFE was developed via a collaborative process involving patients, clinicians, implementation scientists, and health literacy experts. Prototyping was informed by AHRQ low literacy principles and key themes about HCQ use from six patient & clinician focus groups. Experts (n=4) reviewed the first prototype and guided an iterative process to revise subsequent prototypes which were then reviewed and endorsed by patients (n=11) and clinicians (n=9). The final decision aid was implemented in four clinics during 40 visits to examine usability and feasibility. Usability was measured by patient-reported understanding of HCQ's role in SLE after using HCQ-SAFE, and resolution of any decisional conflicts. We used a Likert scale to measure users' likelihood to recommend HCQ-SAFE (NPS= net promoter score). We included "Extremely or very likely" as promoters and "extremely or very unlikely" as detractors and calculated NPS = %promoters – %detractors. Next, feasibility was determined by measuring percentage of visits using HCQ-SAFE, percent completed by a non-MD, and time spent to review HCQ-SAFE.
Results: The final HCQ-SAFE evidence-based shared decision-making laminated tool has data organized using pictograms and plain language across four risks of interest: a) organ damage, b) premature death, c) blood clots, & d) eye toxicity in HCQ users vs. non-users.
During visits a patient and clinician first review pictograms of organ damage risk in SLE, then see how risks decrease with HCQ (Fig 1A). Next, declines in risks of early death and blood clots in HCQ users (Fig 2A-B) vs. low risk of eye toxicity are reviewed (Fig 1B & circles in Fig 1A-2).
We used HCQ-SAFE during 40 patient visits, including 25% non-English speaking patients. All patients reported improved knowledge about damage-free survival benefits of HCQ in SLE (Table 1). Moreover, HCQ-SAFE increased patients requesting an eye referral for HCQ screening by 25%. Decisional conflicts were noted in 26% of visits that all resolved using HCQ-SAFE. HCQ-SAFE garnered high clinician & patient support and high likelihood to recommend (NPS = 100%; avg Likert score = 8.5). Finally, 97% of clinicians reported spending < 10 mins. to review HCQ-SAFE; 60% of discussions were led by a non-MD.
Conclusion: We created HCQ-SAFE, an evidence-based, feasible shared decision-making tool with patients and experts. HCQ-SAFE aims to enhance communication to improve knowledge, clarify misbeliefs, and engage patients in treatment decisions, including those with limited health literacy and proficiency in English.
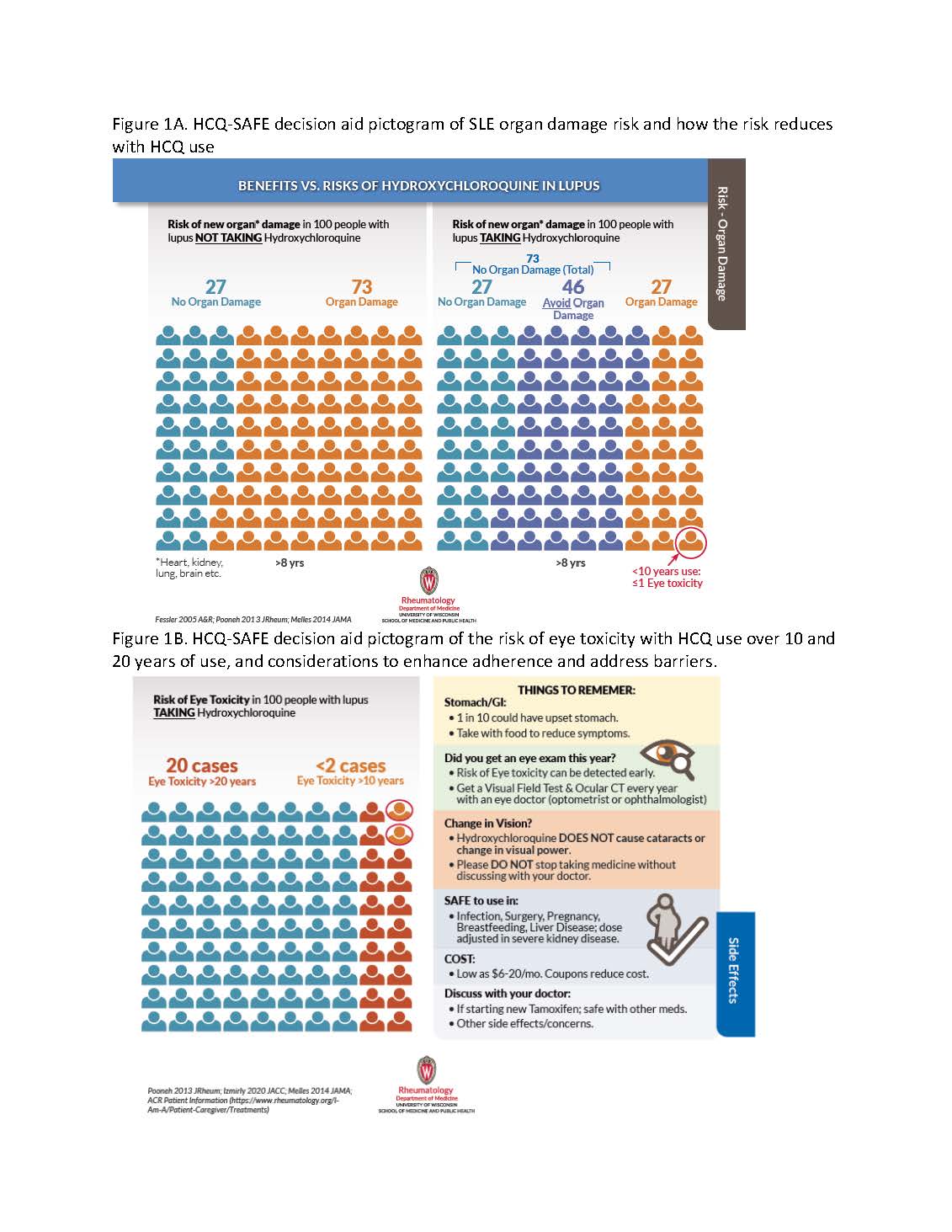 Figure 1A. HCQ-SAFE decision aid pictogram of SLE organ damage risk and how the risk reduces with HCQ use; Figure 1B. HCQ-SAFE decision aid pictogram of the risk of eye toxicity with HCQ use over 10 and 20 years of use, and considerations to enhance adherence and address barriers.
Figure 1A. HCQ-SAFE decision aid pictogram of SLE organ damage risk and how the risk reduces with HCQ use; Figure 1B. HCQ-SAFE decision aid pictogram of the risk of eye toxicity with HCQ use over 10 and 20 years of use, and considerations to enhance adherence and address barriers.
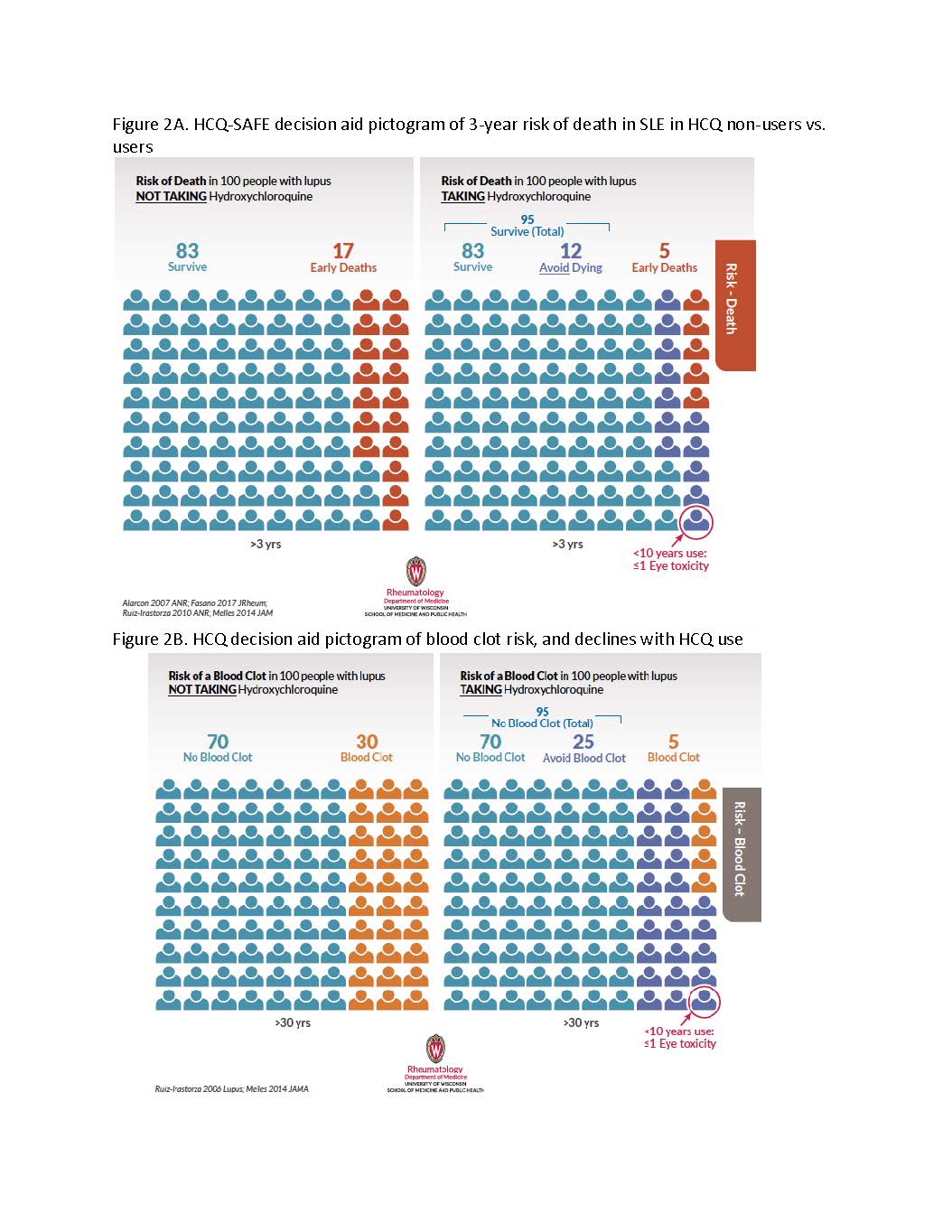 Figure 2A. HCQ-SAFE decision aid pictogram of 3-year risk of death in SLE in HCQ non-users vs. users; Figure 2B. HCQ decision aid pictogram of blood clot risk, and declines with HCQ use
Figure 2A. HCQ-SAFE decision aid pictogram of 3-year risk of death in SLE in HCQ non-users vs. users; Figure 2B. HCQ decision aid pictogram of blood clot risk, and declines with HCQ use
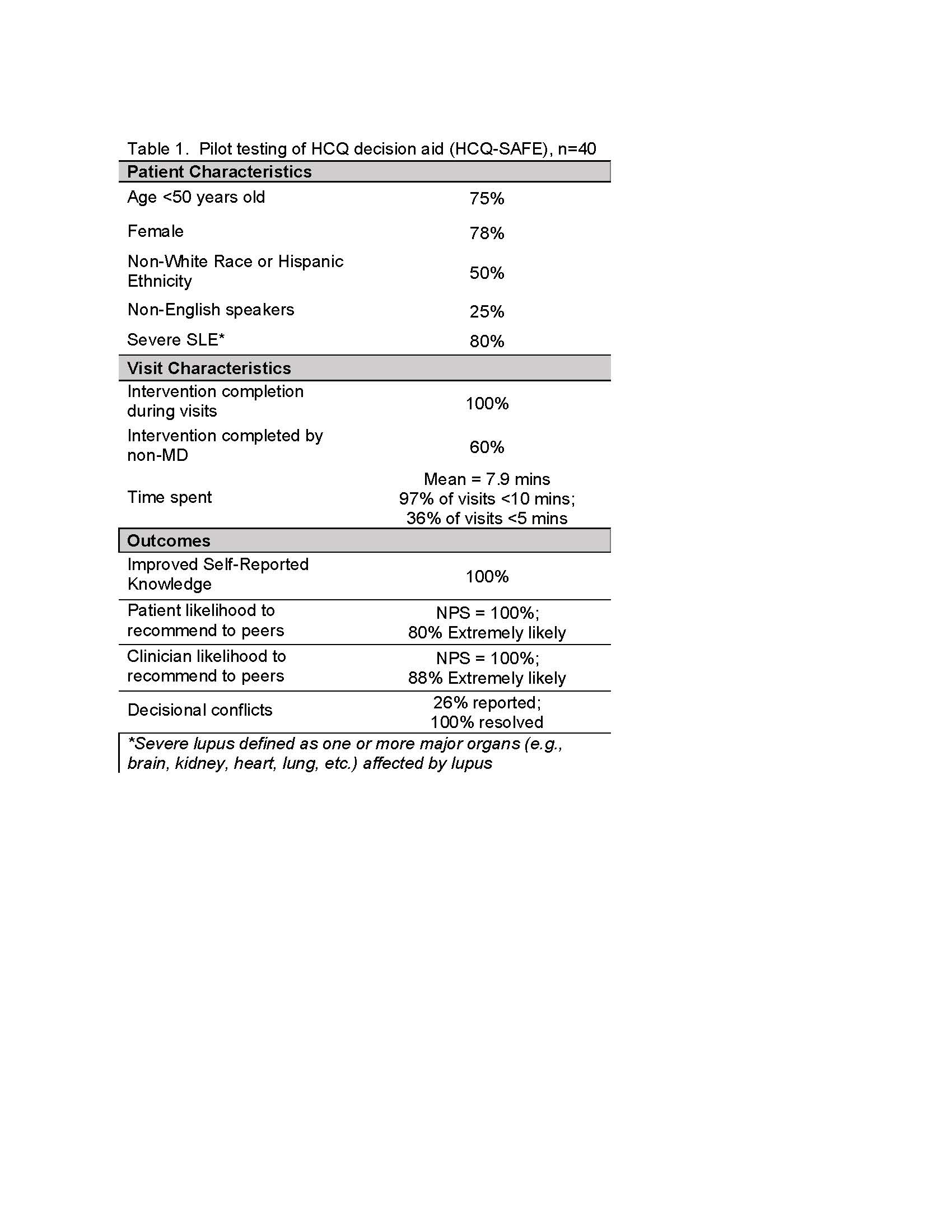 Table 1. Pilot testing of HCQ decision aid (HCQ-SAFE), n=40
Table 1. Pilot testing of HCQ decision aid (HCQ-SAFE), n=40
Disclosures: S. Garg, None; S. Ferguson, None; B. Chewning, None; S. Gomez, None; J. Keevil, None; C. Bartels, Pfizer.
Background/Purpose: Studies report ~83% of SLE patients discontinue hydroxychloroquine (HCQ) and many report suboptimal shared decision-making with their healthcare team. Moreover, patients report knowledge gaps on role of HCQ in improving survival in SLE, and inflated concerns about rare eye toxicity. In complex diseases, involving patients as partners to make therapy decisions can improve adherence and outcomes. Further, AHRQ calls for interventions meeting low health literacy standards. The objective was to describe the development and piloting of a decision aid (HCQ-SAFE) to facilitate shared decision making about HCQ's safety and efficacy.
Methods: Based on prior decision aid development, HCQ-SAFE was developed via a collaborative process involving patients, clinicians, implementation scientists, and health literacy experts. Prototyping was informed by AHRQ low literacy principles and key themes about HCQ use from six patient & clinician focus groups. Experts (n=4) reviewed the first prototype and guided an iterative process to revise subsequent prototypes which were then reviewed and endorsed by patients (n=11) and clinicians (n=9). The final decision aid was implemented in four clinics during 40 visits to examine usability and feasibility. Usability was measured by patient-reported understanding of HCQ's role in SLE after using HCQ-SAFE, and resolution of any decisional conflicts. We used a Likert scale to measure users' likelihood to recommend HCQ-SAFE (NPS= net promoter score). We included "Extremely or very likely" as promoters and "extremely or very unlikely" as detractors and calculated NPS = %promoters – %detractors. Next, feasibility was determined by measuring percentage of visits using HCQ-SAFE, percent completed by a non-MD, and time spent to review HCQ-SAFE.
Results: The final HCQ-SAFE evidence-based shared decision-making laminated tool has data organized using pictograms and plain language across four risks of interest: a) organ damage, b) premature death, c) blood clots, & d) eye toxicity in HCQ users vs. non-users.
During visits a patient and clinician first review pictograms of organ damage risk in SLE, then see how risks decrease with HCQ (Fig 1A). Next, declines in risks of early death and blood clots in HCQ users (Fig 2A-B) vs. low risk of eye toxicity are reviewed (Fig 1B & circles in Fig 1A-2).
We used HCQ-SAFE during 40 patient visits, including 25% non-English speaking patients. All patients reported improved knowledge about damage-free survival benefits of HCQ in SLE (Table 1). Moreover, HCQ-SAFE increased patients requesting an eye referral for HCQ screening by 25%. Decisional conflicts were noted in 26% of visits that all resolved using HCQ-SAFE. HCQ-SAFE garnered high clinician & patient support and high likelihood to recommend (NPS = 100%; avg Likert score = 8.5). Finally, 97% of clinicians reported spending < 10 mins. to review HCQ-SAFE; 60% of discussions were led by a non-MD.
Conclusion: We created HCQ-SAFE, an evidence-based, feasible shared decision-making tool with patients and experts. HCQ-SAFE aims to enhance communication to improve knowledge, clarify misbeliefs, and engage patients in treatment decisions, including those with limited health literacy and proficiency in English.
 Figure 1A. HCQ-SAFE decision aid pictogram of SLE organ damage risk and how the risk reduces with HCQ use; Figure 1B. HCQ-SAFE decision aid pictogram of the risk of eye toxicity with HCQ use over 10 and 20 years of use, and considerations to enhance adherence and address barriers.
Figure 1A. HCQ-SAFE decision aid pictogram of SLE organ damage risk and how the risk reduces with HCQ use; Figure 1B. HCQ-SAFE decision aid pictogram of the risk of eye toxicity with HCQ use over 10 and 20 years of use, and considerations to enhance adherence and address barriers. Figure 2A. HCQ-SAFE decision aid pictogram of 3-year risk of death in SLE in HCQ non-users vs. users; Figure 2B. HCQ decision aid pictogram of blood clot risk, and declines with HCQ use
Figure 2A. HCQ-SAFE decision aid pictogram of 3-year risk of death in SLE in HCQ non-users vs. users; Figure 2B. HCQ decision aid pictogram of blood clot risk, and declines with HCQ use Table 1. Pilot testing of HCQ decision aid (HCQ-SAFE), n=40
Table 1. Pilot testing of HCQ decision aid (HCQ-SAFE), n=40Disclosures: S. Garg, None; S. Ferguson, None; B. Chewning, None; S. Gomez, None; J. Keevil, None; C. Bartels, Pfizer.

